Identification and Characterization of Edible Cricket Peptides on Hypertensive and Glycemic In Vitro Inhibition and Their Anti-Inflammatory Activity on RAW 264.7 Macrophage Cells
- PMID: 33238450
- PMCID: PMC7700588
- DOI: 10.3390/nu12113588
Identification and Characterization of Edible Cricket Peptides on Hypertensive and Glycemic In Vitro Inhibition and Their Anti-Inflammatory Activity on RAW 264.7 Macrophage Cells
Abstract
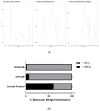
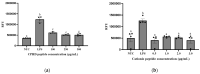
Recent studies continue to demonstrate the potential of edible insects as a protein base to obtain bioactive peptides applicable for functional food development. This study aimed at identifying antihypertensive, anti-glycemic, and anti-inflammatory peptides derived from the in vitro gastrointestinal digests of cricket protein hydrolysates. After sequential fractionation, the protein digest subfraction containing the lowest molecular weight (<0.5 kDa), hydrophobic (C18) and cationic peptides (IEX) was found responsible for the most bioactivity. The cationic peptide fraction significantly reduced (p < 0.05) α-amylase, α-glucosidase, and angiotensin converting enzyme (ACE) activity in vitro, and also inhibited the expression of NF-κB in RAW 264.7 macrophage cells. A total of 28 peptides were identified with mass spectrometry (LC-MS/MS) and de novo sequencing from the potent fraction. Three novel peptides YKPRP, PHGAP, and VGPPQ were chosen for the molecular docking studies. PHGAP and VGPPQ exhibited a higher degree of non-covalent interactions with the enzyme active site residues and binding energies comparable to captopril. Results from this study demonstrate the bioactive potential of edible cricket peptides, especially as ACE inhibitors.
Keywords: ACE inhibition; cationic peptides; cricket protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Impact of commercial precooking of common bean (Phaseolus vulgaris) on the generation of peptides, after pepsin-pancreatin hydrolysis, capable to inhibit dipeptidyl peptidase-IV.J Food Sci. 2015 Jan;80(1):H188-98. doi: 10.1111/1750-3841.12726. Epub 2014 Dec 11. J Food Sci. 2015. PMID: 25495131
-
Production and characterization of anti-hypertensive and anti-diabetic peptides from fermented sheep milk with anti-inflammatory activity: in vitro and molecular docking studies.J Sci Food Agric. 2025 Jun;105(8):4096-4120. doi: 10.1002/jsfa.13617. Epub 2024 Jun 10. J Sci Food Agric. 2025. PMID: 38855927
-
Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein.Food Chem. 2018 Oct 1;262:39-47. doi: 10.1016/j.foodchem.2018.04.058. Epub 2018 Apr 22. Food Chem. 2018. PMID: 29751919
-
Structural characteristic and molecular docking simulation of fish protein-derived peptides: Recent updates on antioxidant, anti-hypertensive and anti-diabetic peptides.Food Chem. 2023 Mar 30;405(Pt A):134737. doi: 10.1016/j.foodchem.2022.134737. Epub 2022 Oct 28. Food Chem. 2023. PMID: 36335734 Review.
-
Bioactive Peptide Discovery from Edible Insects for Potential Applications in Human Health and Agriculture.Molecules. 2023 Jan 27;28(3):1233. doi: 10.3390/molecules28031233. Molecules. 2023. PMID: 36770900 Free PMC article. Review.
Cited by
-
Insects as Valuable Sources of Protein and Peptides: Production, Functional Properties, and Challenges.Foods. 2023 Nov 24;12(23):4243. doi: 10.3390/foods12234243. Foods. 2023. PMID: 38231647 Free PMC article. Review.
-
Edible Insects as a Novel Source of Bioactive Peptides: A Systematic Review.Foods. 2023 May 17;12(10):2026. doi: 10.3390/foods12102026. Foods. 2023. PMID: 37238844 Free PMC article. Review.
-
Stability of Fly Maggot Peptides and Its Alleviating Effect on Lipopolysaccharide Combined with Hemocoagulase Oxidative Stress in Arbor Acres Chicks.Vet Sci. 2024 Oct 1;11(10):470. doi: 10.3390/vetsci11100470. Vet Sci. 2024. PMID: 39453062 Free PMC article.
-
Uncovering the Potential Somatic Angiotensin-Converting Enzyme (sACE) Inhibitory Capacity of Peptides from Acheta domesticus: Insights from In Vitro Gastrointestinal Digestion.Foods. 2024 Oct 29;13(21):3462. doi: 10.3390/foods13213462. Foods. 2024. PMID: 39517245 Free PMC article.
-
Nutritional Characteristics of New Generation Extruded Snack Pellets with Edible Cricket Flour Processed at Various Extrusion Conditions.Antioxidants (Basel). 2023 Jun 10;12(6):1253. doi: 10.3390/antiox12061253. Antioxidants (Basel). 2023. PMID: 37371983 Free PMC article.
References
-
- Estruch R., Martínez-González M.Á., Corella D., Salas-Salvadó J., Ruiz-Gutiérrez V., Covas M.I., Fiol M., Gómez-Gracia E., López-Sabater M.C., Vinyoles E., et al. Effects of a Mediterranean-Style Diet on Cardiovascular Risk Factors: A randomized trial. Ann. Intern. Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. - DOI - PubMed
-
- Luna G.C., Martin-Gonzalez F.S., Mauer L., Liceaga A.M. Cricket (Acheta domesticus) protein hydrolysates’ impact on the physicochemical, structural and sensory properties of tortillas and tortilla chips. J. Insects Food Feed. 2020:1–12. doi: 10.3920/JIFF2020.0010. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

