Ex Vivo Culture Models to Indicate Therapy Response in Head and Neck Squamous Cell Carcinoma
- PMID: 33238461
- PMCID: PMC7700693
- DOI: 10.3390/cells9112527
Ex Vivo Culture Models to Indicate Therapy Response in Head and Neck Squamous Cell Carcinoma
Abstract
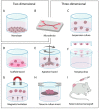
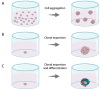
Head and neck squamous cell carcinoma (HNSCC) is characterized by a poor 5 year survival and varying response rates to both standard-of-care and new treatments. Despite advances in medicine and treatment methods, mortality rates have hardly decreased in recent decades. Reliable patient-derived tumor models offer the chance to predict therapy response in a personalized setting, thereby improving treatment efficacy by identifying the most appropriate treatment regimen for each patient. Furthermore, ex vivo tumor models enable testing of novel therapies before introduction in clinical practice. A literature search was performed to identify relevant literature describing three-dimensional ex vivo culture models of HNSCC to examine sensitivity to chemotherapy, radiotherapy, immunotherapy and targeted therapy. We provide a comprehensive overview of the currently used three-dimensional ex vivo culture models for HNSCC with their advantages and limitations, including culture success percentage and comparison to the original tumor. Furthermore, we evaluate the potential of these models to predict patient therapy response.
Keywords: 3D cell culture; drug response; ex vivo model; head and neck cancer; histoculture; organoid; personalized therapy; preclinical prediction model; primary cell culture; sensitivity testing.
Conflict of interest statement
Imke Demers and Johan Donkers declare no conflict of interest. Bernd Kremer reports grants from Pfizer and Novartis. Ernst Jan Speel reports grants from Pfizer and Novartis and honoraria from BMS. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

