Cardioprotection via Metabolism for Rat Heart Preservation Using the High-Pressure Gaseous Mixture of Carbon Monoxide and Oxygen
- PMID: 33238497
- PMCID: PMC7700337
- DOI: 10.3390/ijms21228858
Cardioprotection via Metabolism for Rat Heart Preservation Using the High-Pressure Gaseous Mixture of Carbon Monoxide and Oxygen
Abstract
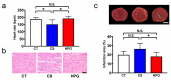
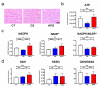
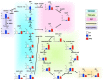
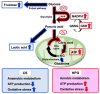
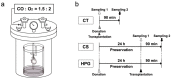
The high-pressure gas (HPG) method with carbon monoxide (CO) and oxygen (O2) mixture maintains the preserved rat heart function. The metabolites of rat hearts preserved using the HPG method (HPG group) and cold storage (CS) method (CS group) by immersion in a stock solution for 24 h were assessed to confirm CO and O2 effects. Lactic acid was significantly lower and citric acid was significantly higher in the HPG group than in the CS group. Moreover, adenosine triphosphate (ATP) levels as well as some pentose phosphate pathway (PPP) metabolites and reduced nicotinamide adenine dinucleotide phosphate (NADPH) were significantly higher in the HPG group than in the CS group. Additionally, reduced glutathione (GSH), which protects cells from oxidative stress, was also significantly higher in the HPG group than in the CS group. These results indicated that each gas, CO and O2, induced the shift from anaerobic to aerobic metabolism, maintaining the energy of ischemic preserved organs, shifting the glucose utilization from glycolysis toward PPP, and reducing oxidative stress. Both CO and O2 in the HPG method have important effects on the ATP supply and decrease oxidative stress for preventing ischemic injury. The HPG method may be useful for clinical application.
Keywords: carbon monoxide; cardioprotection; high-pressure gas; ischemic injury; metabolomics; oxygen; rat; transplantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ardehali A., Esmailian F., Deng M., Soltesz E., Hsich E., Naka Y., Mancini D., Camacho M., Zucker M., Leprince P., et al. Ex-vivoperfusionof donor hearts for human heart transplantation (PROCEED II): A prospective, open- label, multicentre, randomised non-inferiority trial. Lancet. 2015;385:2577–2584. doi: 10.1016/S0140-6736(15)60261-6. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

