Role of Bioactive Peptide Sequences in the Potential Impact of Dairy Protein Intake on Metabolic Health
- PMID: 33238654
- PMCID: PMC7700308
- DOI: 10.3390/ijms21228881
Role of Bioactive Peptide Sequences in the Potential Impact of Dairy Protein Intake on Metabolic Health
Abstract
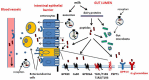
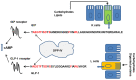
For years, there has been an increasing move towards elucidating the complexities of how food can interplay with the signalling networks underlying energy homeostasis and glycaemic control. Dairy foods can be regarded as the greatest source of proteins and peptides with various health benefits and are a well-recognized source of bioactive compounds. A number of dairy protein-derived peptide sequences with the ability to modulate functions related to the control of food intake, body weight gain and glucose homeostasis have been isolated and characterized. Their being active in vivo may be questionable mainly due to expected low bioavailability after ingestion, and hence their real contribution to the metabolic impact of dairy protein intake needs to be discussed. Some reports suggest that the differential effects of dairy proteins-in particular whey proteins-on mechanisms underlying energy balance and glucose-homeostasis may be attributed to their unique amino acid composition and hence the release of free amino acid mixtures enriched in essential amino acids (i.e., branched-chain-amino acids) upon digestion. Actually, the research reports reviewed in this article suggest that, among a number of dairy protein-derived peptides isolated and characterized as bioactive compounds in vitro, some peptides can be active in vivo post-oral administration through a local action in the gut, or, alternatively, a systemic action on specific molecular targets after entering the systemic circulation. Moreover, these studies highlight the importance of the enteroendocrine system in the cross talk between food proteins and the neuroendocrine network regulating energy balance.
Keywords: DPP-IV activity; appetite and satiety; bioactive peptides; enteroendocrine hormones; food intake; food peptides; glucose homeostasis; identification and characterization; whey.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: a review of the evidence from controlled clinical trials.Adv Nutr. 2013 Jul 1;4(4):418-38. doi: 10.3945/an.113.003723. Adv Nutr. 2013. PMID: 23858091 Free PMC article. Review.
-
Whey proteins in the regulation of food intake and satiety.J Am Coll Nutr. 2007 Dec;26(6):704S-12S. doi: 10.1080/07315724.2007.10719651. J Am Coll Nutr. 2007. PMID: 18187437 Review.
-
Effects of dairy protein and fat on the metabolic syndrome and type 2 diabetes.Rev Diabet Stud. 2014 Summer;11(2):153-66. doi: 10.1900/RDS.2014.11.153. Epub 2014 Aug 10. Rev Diabet Stud. 2014. PMID: 25396403 Free PMC article. Review.
-
Effect of milk protein intake and casein-to-whey ratio in breakfast meals on postprandial glucose, satiety ratings, and subsequent meal intake.J Dairy Sci. 2018 Oct;101(10):8688-8701. doi: 10.3168/jds.2018-14419. Epub 2018 Aug 20. J Dairy Sci. 2018. PMID: 30139624 Clinical Trial.
-
Plasma Free Amino Acid Responses to Whey Protein and Their Relationships with Gastric Emptying, Blood Glucose- and Appetite-Regulatory Hormones and Energy Intake in Lean Healthy Men.Nutrients. 2019 Oct 15;11(10):2465. doi: 10.3390/nu11102465. Nutrients. 2019. PMID: 31618863 Free PMC article. Clinical Trial.
Cited by
-
Effects of Bioactive Peptides from Atlantic Salmon Processing By-Products on Oxyntopeptic and Enteroendocrine Cells of the Gastric Mucosa of European Seabass and Gilthead Seabream.Animals (Basel). 2023 Sep 26;13(19):3020. doi: 10.3390/ani13193020. Animals (Basel). 2023. PMID: 37835626 Free PMC article.
-
In Vivo and In Vitro Comparison of the DPP-IV Inhibitory Potential of Food Proteins from Different Origins after Gastrointestinal Digestion.Int J Mol Sci. 2022 Jul 28;23(15):8365. doi: 10.3390/ijms23158365. Int J Mol Sci. 2022. PMID: 35955493 Free PMC article.
-
Difficulties in Establishing the Adverse Effects of β-Casomorphin-7 Released from β-Casein Variants-A Review.Foods. 2023 Aug 22;12(17):3151. doi: 10.3390/foods12173151. Foods. 2023. PMID: 37685085 Free PMC article. Review.
-
A2 Milk and BCM-7 Peptide as Emerging Parameters of Milk Quality.Front Nutr. 2022 Apr 27;9:842375. doi: 10.3389/fnut.2022.842375. eCollection 2022. Front Nutr. 2022. PMID: 35571904 Free PMC article. Review.
-
Effect of Ripening and In Vitro Digestion on Bioactive Peptides Profile in Ras Cheese and Their Biological Activities.Biology (Basel). 2023 Jul 2;12(7):948. doi: 10.3390/biology12070948. Biology (Basel). 2023. PMID: 37508379 Free PMC article.
References
-
- Sanchez A., Vazquez A. Bioactive peptides: A review. Food Qual. Saf. 2017;1:29–46. doi: 10.1093/fqs/fyx006. - DOI
-
- Korhonen H., Pihlanto A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006;16:945–969. doi: 10.1016/j.idairyj.2005.10.012. - DOI
-
- Hernandez-Ledesma B., Garcia-Nebot M.J., Fernandez-Tomè S., Amigo L., Recio I. Dairy protein hydrolysates: Peptides for health benefits. Int. Dairy J. 2014;38:82–100. doi: 10.1016/j.idairyj.2013.11.004. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

