Outcome quality standards in advanced ovarian cancer surgery
- PMID: 33239057
- PMCID: PMC7690155
- DOI: 10.1186/s12957-020-02064-7
Outcome quality standards in advanced ovarian cancer surgery
Erratum in
-
Correction to: Outcome quality standards in advanced ovarian cancer surgery.World J Surg Oncol. 2020 Dec 7;18(1):323. doi: 10.1186/s12957-020-02102-4. World J Surg Oncol. 2020. PMID: 33287840 Free PMC article.
Abstract
Introduction: Advanced ovarian cancer surgery (AOCS) frequently results in serious postoperative complications. Because managing AOCS is difficult, some standards need to be established that allow surgeons to assess the quality of treatment provided and consider what aspects should improve. This study aimed to identify quality indicators (QIs) of clinical relevance and to establish their acceptable quality limits (i.e., standard) in AOCS.
Materials and methods: We performed a systematic search on clinical practice guidelines, consensus conferences, and reviews on the outcome and quality of AOCS to identify which QIs have clinical relevance in AOCS. We then searched the literature (from January 2006 to December 2018) for each QI in combination with the keywords of advanced ovarian cancer, surgery, outcome, and oncology. Standards for each QI were determined by statistical process control techniques. The acceptable quality limits for each QI were defined as being within the limits of the 99.8% interval, which indicated a favorable outcome.
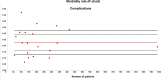
Results: A total of 38 studies were included. The QIs selected for AOCS were complete removal of the tumor upon visual inspection (complete cytoreductive surgery), a residual tumor of < 1 cm (optimal cytoreductive surgery), a residual tumor of > 1 cm (suboptimal cytoreductive surgery), major morbidity, and 5-year survival. The rates of complete cytoreductive surgery, optimal cytoreductive surgery, suboptimal cytoreductive surgery, morbidity, and 5-year survival had quality limits of < 27%, < 23%, > 39%, > 33%, and < 27%, respectively.
Conclusion: Our results provide a general view of clinical indicators for AOCS. Acceptable quality limits that can be considered as standards were established.
Keywords: Advanced ovarian cancer; Cytoreductive surgery; Medical care; Morbidity; Outcome; Quality indicator; Tumor.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Lavoue V, Huchon C, Akladios C, et al. Management of epithelial cancer of the ovary, fallopian tube, and primary peritoneum. Short text of the French Clinical Practice Guidelines issued by FRANCOGYN, CNGOF, SFOG, and GINECO-ARCAGY, and endorsed by INCa. Eur J Obstet Gynecol Reprod Biol. 2019;236:214–223. doi: 10.1016/j.ejogrb.2019.03.010. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

