Impact of a package of diagnostic tools, clinical algorithm, and training and communication on outpatient acute fever case management in low- and middle-income countries: protocol for a randomized controlled trial
- PMID: 33239106
- PMCID: PMC7687811
- DOI: 10.1186/s13063-020-04897-9
Impact of a package of diagnostic tools, clinical algorithm, and training and communication on outpatient acute fever case management in low- and middle-income countries: protocol for a randomized controlled trial
Abstract
Background: The management of acute febrile illnesses places a heavy burden on clinical services in many low- and middle-income countries (LMICs). Bacterial and viral aetiologies of acute fevers are often clinically indistinguishable and, in the absence of diagnostic tests, the 'just-in-case' use of antibiotics by many health workers has become common practice, which has an impact on drug-resistant infections. Our study aims to answer the following question: in patients with undifferentiated febrile illness presenting to outpatient clinics/peripheral health centres in LMICs, can we demonstrate an improvement in clinical outcomes and reduce unnecessary antibiotic prescription over current practice by using a combination of simple, accurate diagnostic tests, clinical algorithms, and training and communication (intervention package)?
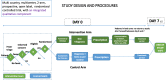
Methods: We designed a randomized, controlled clinical trial to evaluate the impact of our intervention package on clinical outcomes and antibiotic prescription rates in acute febrile illnesses. Available, point-of-care, pathogen-specific and non-pathogen specific (host markers), rapid diagnostic tests (RDTs) included in the intervention package were selected based on pre-defined criteria. Nine clinical study sites in six countries (Burkina Faso, Ghana, India, Myanmar, Nepal and Uganda), which represent heterogeneous outpatient care settings, were selected. We considered the expected seasonal variations in the incidence of acute febrile illnesses across all the sites by ensuring a recruitment period of 12 months. A master protocol was developed and adapted for country-specific ethical submissions. Diagnostic algorithms and choice of RDTs acknowledged current data on aetiologies of acute febrile illnesses in each country. We included a qualitative evaluation of drivers and/or deterrents of uptake of new diagnostics and antibiotic use for acute febrile illnesses. Sample size estimations were based on historical site data of antibiotic prescription practices for malarial and non-malarial acute fevers. Overall, 9 semi-independent studies will enrol a minimum of 21,876 patients and an aggregate data meta-analysis will be conducted on completion.
Discussion: This study is expected to generate vital evidence needed to inform policy decisions on the role of rapid diagnostic tests in the clinical management of acute febrile illnesses, with a view to controlling the rise of antimicrobial resistance in LMICs.
Trial registration: Clinicaltrials.gov NCT04081051 . Registered on 6 September 2019. Protocol version 1.4 dated 20 December 2019.
Keywords: Antibiotic prescription; Antimicrobial resistance; Febrile illness; Outpatient fever management; Randomized controlled trial.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- World Health Organization. Antimicrobial resistance: global report on surveillance. World Health Organization; 2014. Available from: http://apps.who.int/iris/handle/10665/112642. Accessed 1 May 2020.
-
- O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Rev Antimicrob Resist. 2014. http://amrreview.org/Publications.
-
- Global action plan on antimicrobial resistance: World Health Organization; 2015. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

