Characterization of carp seminal plasma Wap65-2 and its participation in the testicular immune response and temperature acclimation
- PMID: 33239112
- PMCID: PMC7688007
- DOI: 10.1186/s13567-020-00858-x
Characterization of carp seminal plasma Wap65-2 and its participation in the testicular immune response and temperature acclimation
Abstract
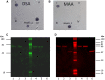
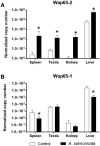
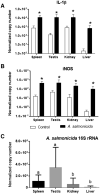
Two functionally distinct isoforms of warm-temperature acclimation related 65-kDa protein (Wap65-1 and Wap65-2) with a role in the immune response are present in fish. To our knowledge, contrary to Wap65-1, Wap65-2 has neither been isolated nor functionally characterized in carp especially in reproductive system. The aim of this study was to characterize Wap65-2 and ascertain its functions in immune response and temperature acclimation within reproductive system. Wap65-2 corresponded to one of the most abundant proteins in carp seminal plasma, with a high immunologic similarity to their counterparts in seminal plasma of other fish species and a wide tissue distribution, with predominant expression in the liver. The immunohistochemical localization of Wap65-2 to spermatogonia, Leydig cells, and the epithelium of blood vessels within the testis suggests its role in iron metabolism during spermatogenesis and maintenance of blood-testis barrier integrity. Wap65-2 secretion by the epithelial cells of the spermatic duct and its presence around spermatozoa suggests its involvement in the protection of spermatozoa against damage caused by heme released from erythrocytes following hemorrhage and inflammation. Our results revealed an isoform-specific response of Wap65 to temperature acclimation and Aeromonas salmonicida infection which alters blood-testis barrier integrity. Wap65-2 seems to be related to the immune response against bacteria, while Wap65-1 seems to be involved in temperature acclimation. This study expands the understanding of the mechanism of carp testicular immunity against bacterial challenge and temperature changes, in which Wap65-2 seems to be involved and highlights their potential usefulness as biomarkers of inflammation and temperature acclimation.
Keywords: Wap65; acclimation; cDNA; fish; hemopexin; infection; reproductive system; semen.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures








Similar articles
-
Modulation of warm-temperature-acclimation-associated 65-kDa protein genes (Wap65-1 and Wap65-2) in mud loach (Misgurnus mizolepis, Cypriniformes) liver in response to different stimulatory treatments.Fish Shellfish Immunol. 2012 May;32(5):662-9. doi: 10.1016/j.fsi.2012.01.009. Epub 2012 Feb 2. Fish Shellfish Immunol. 2012. PMID: 22326761
-
The warm temperature acclimation protein (Wap65) has an important role in the inflammatory response of turbot (Scophthalmus maximus).Fish Shellfish Immunol. 2014 Nov;41(1):80-92. doi: 10.1016/j.fsi.2014.04.012. Epub 2014 Apr 30. Fish Shellfish Immunol. 2014. PMID: 24794581
-
Identification and characterization of warm temperature acclimation proteins (Wap65s) in rainbow trout (Oncorhynchus mykiss).Dev Comp Immunol. 2022 Oct;135:104475. doi: 10.1016/j.dci.2022.104475. Epub 2022 Jun 19. Dev Comp Immunol. 2022. PMID: 35732223
-
The warm temperature acclimation protein Wap65 as an immune response gene: its duplicates are differentially regulated by temperature and bacterial infections.Mol Immunol. 2008 Mar;45(5):1458-69. doi: 10.1016/j.molimm.2007.08.012. Epub 2007 Oct 24. Mol Immunol. 2008. PMID: 17920125
-
Identification of warm temperature acclimation-associated 65-kDa protein-2 in Kumgang fat minnow Rhynchocypris kumgangensis.J Exp Zool A Ecol Integr Physiol. 2017 Dec;327(10):611-619. doi: 10.1002/jez.2149. Epub 2018 Mar 15. J Exp Zool A Ecol Integr Physiol. 2017. PMID: 29542267
Cited by
-
Secreted novel AID/APOBEC-like deaminase 1 (SNAD1) - a new important player in fish immunology.Front Immunol. 2024 Mar 27;15:1340273. doi: 10.3389/fimmu.2024.1340273. eCollection 2024. Front Immunol. 2024. PMID: 38601149 Free PMC article. Review.
-
Comparative proteomic analysis of the ovarian fluid and eggs of Siberian sturgeon.BMC Genomics. 2024 May 7;25(1):451. doi: 10.1186/s12864-024-10309-y. BMC Genomics. 2024. PMID: 38714919 Free PMC article.
References
-
- Balla G, Vercellotti GM, Muller-Eberhard U, Eaton J, Jacob HS. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab Invest. 1991;64:648–655. - PubMed
-
- Watabe S, Kikuchi K, Aida K. Cold- and warm-temperature acclimation induces specific cytosolic protein in goldfish and carp. Nippon Suisan Gakkaish. 1993;59:151e6. doi: 10.2331/suisan.59.151. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

