Hypothesis: Alpha-1-antitrypsin is a promising treatment option for COVID-19
- PMID: 33239231
- PMCID: PMC7659642
- DOI: 10.1016/j.mehy.2020.110394
Hypothesis: Alpha-1-antitrypsin is a promising treatment option for COVID-19
Abstract
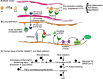
No definitive treatment for COVID-19 exists although promising results have been reported with remdesivir and glucocorticoids. Short of a truly effective preventive or curative vaccine against SARS-CoV-2, it is becoming increasingly clear that multiple pathophysiologic processes seen with COVID-19 as well as SARS-CoV-2 itself should be targeted. Because alpha-1-antitrypsin (AAT) embraces a panoply of biologic activities that may antagonize several pathophysiologic mechanisms induced by SARS-CoV-2, we hypothesize that this naturally occurring molecule is a promising agent to ameliorate COVID-19. We posit at least seven different mechanisms by which AAT may alleviate COVID-19. First, AAT is a serine protease inhibitor (SERPIN) shown to inhibit TMPRSS-2, the host serine protease that cleaves the spike protein of SARS-CoV-2, a necessary preparatory step for the virus to bind its cell surface receptor ACE2 to gain intracellular entry. Second, AAT has anti-viral activity against other RNA viruses HIV and influenza as well as induces autophagy, a known host effector mechanism against MERS-CoV, a related coronavirus that causes the Middle East Respiratory Syndrome. Third, AAT has potent anti-inflammatory properties, in part through inhibiting both nuclear factor-kappa B (NFκB) activation and ADAM17 (also known as tumor necrosis factor-alpha converting enzyme), and thus may dampen the hyper-inflammatory response of COVID-19. Fourth, AAT inhibits neutrophil elastase, a serine protease that helps recruit potentially injurious neutrophils and implicated in acute lung injury. AAT inhibition of ADAM17 also prevents shedding of ACE2 and hence may preserve ACE2 inhibition of bradykinin, reducing the ability of bradykinin to cause a capillary leak in COVID-19. Fifth, AAT inhibits thrombin, and venous thromboembolism and in situ microthrombi and macrothrombi are increasingly implicated in COVID-19. Sixth, AAT inhibition of elastase can antagonize the formation of neutrophil extracellular traps (NETs), a complex extracellular structure comprised of neutrophil-derived DNA, histones, and proteases, and implicated in the immunothrombosis of COVID-19; indeed, AAT has been shown to change the shape and adherence of non-COVID-19-related NETs. Seventh, AAT inhibition of endothelial cell apoptosis may limit the endothelial injury linked to severe COVID-19-associated acute lung injury, multi-organ dysfunction, and pre-eclampsia-like syndrome seen in gravid women. Furthermore, because both NETs formation and the presence of anti-phospholipid antibodies are increased in both COVID-19 and non-COVID pre-eclampsia, it suggests a similar vascular pathogenesis in both disorders. As a final point, AAT has an excellent safety profile when administered to patients with AAT deficiency and is dosed intravenously once weekly but also comes in an inhaled preparation. Thus, AAT is an appealing drug candidate to treat COVID-19 and should be studied.
Keywords: Anti-inflammation; Anti-thrombosis; NETs; SARS-CoV-2; SERPIN; Serine protease.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Dr. Robert A. Sandhaus is the Medical Director of AlphaNet.
Figures


References
-
- Johnson R.M., Vinetz J.M. Dexamethasone in the management of covid-19: Preliminary trial results are mostly good news, but timing is everything. BMJ. 2020 E-pub. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

