Prescribing practices of lopinavir/ritonavir, hydroxychloroquine and azithromycin during the COVID-19 epidemic crisis and pharmaceutical interventions in a French teaching hospital
- PMID: 33239282
- PMCID: PMC7689541
- DOI: 10.1136/ejhpharm-2020-002449
Prescribing practices of lopinavir/ritonavir, hydroxychloroquine and azithromycin during the COVID-19 epidemic crisis and pharmaceutical interventions in a French teaching hospital
Abstract
Objective: The aims of this study were to describe prescribing practices of lopinavir/ritonavir, hydroxychloroquine and azithromycin during the COVID-19 epidemic crisis (primary endpoint), then to characterise pharmaceutical interventions (PIs) targeted to these medications and evaluate the impact of these PIs on prescribers' practices (secondary end-points).
Methods: This retrospective observational study was carried out at the University Hospital of Strasbourg (France) from March to April 2020. The analysed population excluded patients from intensive care units but included all other adult patients with COVID-19 who received at least one dose of lopinavir/ritonavir combination, hydroxychloroquine or azithromycin, while inpatients. Analyses were performed by using data extracted from electronic medical records.
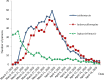
Result: During the study period, 278 patients were included. A rapid decrease in lopinavir/ritonavir prescriptions was observed. This was accompanied by an increase in hydroxychloroquine and azithromycin prescriptions until the end of March, followed by a decrease leading to the disappearance of these two medications in April. The pharmaceutical analysis of the prescriptions resulted in 59 PIs of which 21 were associated with lopinavir/ritonavir, 32 with hydroxychloroquine and 6 with azithromycin. Regarding the medication-related problems, the most frequent ones were incorrect treatment durations (n=32 (54.2%)), drug interactions with potential torsadogenic reactions (n=14 (23.7%)) and incorrect dosing (n=6 (10.2%)). From the 59 PIs, 48 (81.4%) were accepted and physicians adjusted the medication regimens in a timely manner.
Conclusion: This study demonstrated the value-even more meaningful in a crisis situation-of a strong synergy between physicians and pharmacists for patient-safety focused practices.
Keywords: drug misuse; drug-related side effects and adverse reactions; evidence-based medicine; hospital; pharmacy service; quality of health care.
© European Association of Hospital Pharmacists 2021. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Early experimental COVID-19 therapies: associations with length of hospital stay, mortality and related costs.Swiss Med Wkly. 2020 Dec 31;150:w20446. doi: 10.4414/smw.2020.20446. eCollection 2020 Dec 14. Swiss Med Wkly. 2020. PMID: 33382449
-
"Off-label" use of hydroxychloroquine, azithromycin, lopinavir-ritonavir and chloroquine in COVID-19: A survey of cardiac adverse drug reactions by the French Network of Pharmacovigilance Centers.Therapie. 2020 Jul-Aug;75(4):371-379. doi: 10.1016/j.therap.2020.05.002. Epub 2020 May 7. Therapie. 2020. PMID: 32418730 Free PMC article.
-
Lopinavir-ritonavir versus hydroxychloroquine for viral clearance and clinical improvement in patients with mild to moderate coronavirus disease 2019.Korean J Intern Med. 2021 Mar;36(Suppl 1):S253-S263. doi: 10.3904/kjim.2020.224. Epub 2020 Jun 16. Korean J Intern Med. 2021. PMID: 32536150 Free PMC article.
-
Off-label use of chloroquine, hydroxychloroquine, azithromycin and lopinavir/ritonavir in COVID-19 risks prolonging the QT interval by targeting the hERG channel.Eur J Pharmacol. 2021 Feb 15;893:173813. doi: 10.1016/j.ejphar.2020.173813. Epub 2020 Dec 18. Eur J Pharmacol. 2021. PMID: 33345848 Free PMC article. Review.
-
Psychopharmacology of COVID-19.Psychosomatics. 2020 Sep-Oct;61(5):411-427. doi: 10.1016/j.psym.2020.05.006. Epub 2020 May 18. Psychosomatics. 2020. PMID: 32425246 Free PMC article. Review.
Cited by
-
Safety of Short-Term Treatments with Oral Chloroquine and Hydroxychloroquine in Patients with and without COVID-19: A Systematic Review.Pharmaceuticals (Basel). 2022 May 21;15(5):634. doi: 10.3390/ph15050634. Pharmaceuticals (Basel). 2022. PMID: 35631460 Free PMC article. Review.
-
Clinical Pharmacist-Led Medication Review in Hospitalized Confirmed or Probable Patients with COVID-19 During the First Wave of COVID-19 Pandemic.Turk J Pharm Sci. 2024 May 14;21(2):152-158. doi: 10.4274/tjps.galenos.2023.47105. Turk J Pharm Sci. 2024. PMID: 38742835 Free PMC article.
-
Pharmacist-driven antimicrobial stewardship interventions in patients with COVID-19: a scoping review.Int J Clin Pharm. 2023 Jun;45(3):613-621. doi: 10.1007/s11096-023-01574-0. Epub 2023 May 10. Int J Clin Pharm. 2023. PMID: 37162655 Free PMC article.
-
Drug-related problems in patients admitted for SARS-CoV-2 infection during the COVID-19 pandemic.Front Pharmacol. 2022 Nov 24;13:993158. doi: 10.3389/fphar.2022.993158. eCollection 2022. Front Pharmacol. 2022. PMID: 36506516 Free PMC article.
References
-
- Liu S, Luo P, Tang M, et al. . Providing pharmacy services during the coronavirus pandemic. Int J Clin Pharm 2020;42:299–304https://doi.org/10.1007/s11096-020-01017-0 - DOI - PMC - PubMed
-
- Dossier de Presse . Covid-19: Point de situation sur le Grand Est, 2020. Available: https://www.grand-est.ars.sante.fr [Accessed 4 Jul 2020].
-
- National Health Commission . Notice on Issuance of the sixth edition of the guidance for COVID-19: prevention, control, diagnosis, and management, 2020. Available: http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aef... [Accessed 4 Jul 2020].
-
- National Health Commission . Notice on Issuance of the seventh edition of the guidance for COVID-19: prevention, control, diagnosis, and management, 2020. Available: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb19... [Accessed 4 Jul 2020].
-
- Interactions with experimental COVID-19 therapies. University of Liverpool. Available: https://www.covid19-druginteractions.org [Accessed 4 Jul 2020].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous