Redox state of Earth's magma ocean and its Venus-like early atmosphere
- PMID: 33239296
- PMCID: PMC7688334
- DOI: 10.1126/sciadv.abd1387
Redox state of Earth's magma ocean and its Venus-like early atmosphere
Abstract
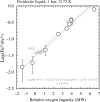
Exchange between a magma ocean and vapor produced Earth's earliest atmosphere. Its speciation depends on the oxygen fugacity (fO2) set by the Fe3+/Fe2+ ratio of the magma ocean at its surface. Here, we establish the relationship between fO2 and Fe3+/Fe2+ in quenched liquids of silicate Earth-like composition at 2173 K and 1 bar. Mantle-derived rocks have Fe3+/(Fe3++Fe2+) = 0.037 ± 0.005, at which the magma ocean defines an fO2 0.5 log units above the iron-wüstite buffer. At this fO2, the solubilities of H-C-N-O species in the magma ocean produce a CO-rich atmosphere. Cooling and condensation of H2O would have led to a prebiotic terrestrial atmosphere composed of CO2-N2, in proportions and at pressures akin to those observed on Venus. Present-day differences between Earth's atmosphere and those of her planetary neighbors result from Earth's heliocentric location and mass, which allowed geologically long-lived oceans, in-turn facilitating CO2 drawdown and, eventually, the development of life.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Hunten D. M., Atmospheric evolution of the terrestrial planets. Science 259, 915–920 (1993).
-
- Lammer H., Zerkle A. L., Gebauer S., Tosi N., Noack L., Scherf M., Pilat-Lohinger E., Güdel M., Grenfell J. L., Godolt M., Nikolaou A., Origin and evolution of the atmospheres of early Venus, Earth and Mars. Astron. Astrophys. Rev. 26, 2 (2018).
-
- Morbidelli A., Lunine J. I., O’Brien D. P., Raymond S. N., Walsh K. J., Building terrestrial planets. Annu. Rev. Earth Planet. Sci. 40, 251–275 (2012).
-
- Marty B., The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth Planet. Sci. Lett. 313–314, 56–66 (2012).
-
- Albarède F., Volatile accretion history of the terrestrial planets and dynamic implications. Nature 461, 1227–1233 (2009). - PubMed
LinkOut - more resources
Full Text Sources

