NAIL: an evolutionarily conserved lncRNA essential for licensing coordinated activation of p38 and NFκB in colitis
- PMID: 33239342
- PMCID: PMC8458091
- DOI: 10.1136/gutjnl-2020-322980
NAIL: an evolutionarily conserved lncRNA essential for licensing coordinated activation of p38 and NFκB in colitis
Abstract
Objective: NFκB is the key modulator in inflammatory disorders. However, the key regulators that activate, fine-tune or shut off NFκB activity in inflammatory conditions are poorly understood. In this study, we aim to investigate the roles that NFκB-specific long non-coding RNAs (lncRNAs) play in regulating inflammatory networks.
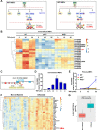
Design: Using the first genetic-screen to identify NFκB-specific lncRNAs, we performed RNA-seq from the p65-/- and Ikkβ-/- mouse embryonic fibroblasts and report the identification of an evolutionary conserved lncRNA designated mNAIL (mice) or hNAIL (human). hNAIL is upregulated in human inflammatory disorders, including UC. We generated mNAILΔNFκB mice, wherein deletion of two NFκB sites in the proximal promoter of mNAIL abolishes its induction, to study its function in colitis.
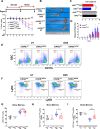
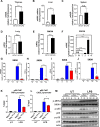
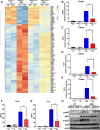
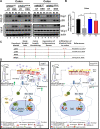
Results: NAIL regulates inflammation via sequestering and inactivating Wip1, a known negative regulator of proinflammatory p38 kinase and NFκB subunit p65. Wip1 inactivation leads to coordinated activation of p38 and covalent modifications of NFκB, essential for its genome-wide occupancy on specific targets. NAIL enables an orchestrated response for p38 and NFκB coactivation that leads to differentiation of precursor cells into immature myeloid cells in bone marrow, recruitment of macrophages to inflamed area and expression of inflammatory genes in colitis.
Conclusion: NAIL directly regulates initiation and progression of colitis and its expression is highly correlated with NFκB activity which makes it a perfect candidate to serve as a biomarker and a therapeutic target for IBD and other inflammation-associated diseases.
Keywords: chronic ulcerative colitis; inflammation; inflammatory bowel disease.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
