The many lives of Myc in the pancreatic β-cell
- PMID: 33239359
- PMCID: PMC7949031
- DOI: 10.1074/jbc.REV120.011149
The many lives of Myc in the pancreatic β-cell
Abstract
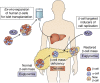
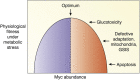
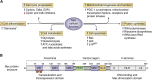
Diabetes results from insufficient numbers of functional pancreatic β-cells. Thus, increasing the number of available functional β-cells ex vivo for transplantation, or regenerating them in situ in diabetic patients, is a major focus of diabetes research. The transcription factor, Myc, discovered decades ago lies at the nexus of most, if not all, known proliferative pathways. Based on this, many studies in the 1990s and early 2000s explored the potential of harnessing Myc expression to expand β-cells for diabetes treatment. Nearly all these studies in β-cells used pathophysiological or supraphysiological levels of Myc and reported enhanced β-cell death, dedifferentiation, or the formation of insulinomas if cooverexpressed with Bcl-xL, an inhibitor of apoptosis. This obviously reduced the enthusiasm for Myc as a therapeutic target for β-cell regeneration. However, recent studies indicate that "gentle" induction of Myc expression enhances β-cell replication without induction of cell death or loss of insulin secretion, suggesting that appropriate levels of Myc could have therapeutic potential for β-cell regeneration. Furthermore, although it has been known for decades that Myc is induced by glucose in β-cells, very little is known about how this essential anabolic transcription factor perceives and responds to nutrients and increased insulin demand in vivo. Here we summarize the previous and recent knowledge of Myc in the β-cell, its potential for β-cell regeneration, and its physiological importance for neonatal and adaptive β-cell expansion.
Keywords: DNA methylation; Myc; adaptation; aging; diabetes; glucose; pancreatic β-cell; proliferation.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Rhodes C.J. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384. - PubMed
-
- Wang P., Fiaschi-Taesch N.M., Vasavada R.C., Scott D.K., Garcia-Ocana A., Stewart A.F. Diabetes mellitus-advances and challenges in human beta-cell proliferation. Nat. Rev. Endocrinol. 2015;11:201–212. - PubMed
-
- Butler P.C., Meier J.J., Butler A.E., Bhushan A. The replication of beta cells in normal physiology, in disease and for therapy. Nat. Clin. Pract. Endocrinol. Metab. 2007;3:758–768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

