Pseudomonas Quinolone Signal-Induced Outer Membrane Vesicles Enhance Biofilm Dispersion in Pseudomonas aeruginosa
- PMID: 33239369
- PMCID: PMC7690959
- DOI: 10.1128/mSphere.01109-20
Pseudomonas Quinolone Signal-Induced Outer Membrane Vesicles Enhance Biofilm Dispersion in Pseudomonas aeruginosa
Abstract
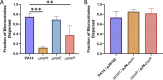
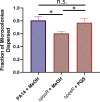
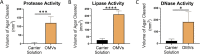
Bacterial biofilms are major contributors to chronic infections in humans. Because they are recalcitrant to conventional therapy, they present a particularly difficult treatment challenge. Identifying factors involved in biofilm development can help uncover novel targets and guide the development of antibiofilm strategies. Pseudomonas aeruginosa causes surgical site, burn wound, and hospital-acquired infections and is also associated with aggressive biofilm formation in the lungs of cystic fibrosis patients. A potent but poorly understood contributor to P. aeruginosa virulence is the ability to produce outer membrane vesicles (OMVs). OMV trafficking has been associated with cell-cell communication, virulence factor delivery, and transfer of antibiotic resistance genes. Because OMVs have almost exclusively been studied using planktonic cultures, little is known about their biogenesis and function in biofilms. Several groups have shown that Pseudomonas quinolone signal (PQS) induces OMV formation in P. aeruginosa Our group described a biophysical mechanism for this and recently showed it is operative in biofilms. Here, we demonstrate that PQS-induced OMV production is highly dynamic during biofilm development. Interestingly, PQS and OMV synthesis are significantly elevated during dispersion compared to attachment and maturation stages. PQS biosynthetic and receptor mutant biofilms were significantly impaired in their ability to disperse, but this phenotype was rescued by genetic complementation or exogenous addition of PQS. Finally, we show that purified OMVs can actively degrade extracellular protein, lipid, and DNA. We therefore propose that enhanced production of PQS-induced OMVs during biofilm dispersion facilitates cell escape by coordinating the controlled degradation of biofilm matrix components.IMPORTANCE Treatments that manipulate biofilm dispersion hold the potential to convert chronic drug-tolerant biofilm infections from protected sessile communities into released populations that are orders-of-magnitude more susceptible to antimicrobial treatment. However, dispersed cells often exhibit increased acute virulence and dissemination phenotypes. A thorough understanding of the dispersion process is therefore critical before this promising strategy can be effectively employed. Pseudomonas quinolone signal (PQS) has been implicated in early biofilm development, but we hypothesized that its function as an outer membrane vesicle (OMV) inducer may contribute at multiple stages. Here, we demonstrate that PQS and OMVs are differentially produced during Pseudomonas aeruginosa biofilm development and provide evidence that effective biofilm dispersion is dependent on the production of PQS-induced OMVs, which likely act as delivery vehicles for matrix-degrading enzymes. These findings lay the groundwork for understanding OMV contributions to biofilm development and suggest a model to explain the controlled matrix degradation that accompanies biofilm dispersion in many species.
Keywords: PQS; Pseudomonas aeruginosa; biofilms; dispersion; outer membrane vesicles; quorum sensing; secretion systems.
Copyright © 2020 Cooke et al.
Figures


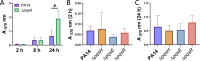

References
-
- Alhede M, Kragh KN, Qvortrup K, Allesen-Holm M, van Gennip M, Christensen LD, Jensen PØ, Nielsen AK, Parsek M, Wozniak D, Molin S, Tolker-Nielsen T, Høiby N, Givskov M, Bjarnsholt T. 2011. Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm. PLoS One 6:e27943. doi: 10.1371/journal.pone.0027943. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
