Evaluating 10 Commercially Available SARS-CoV-2 Rapid Serological Tests by Use of the STARD (Standards for Reporting of Diagnostic Accuracy Studies) Method
- PMID: 33239381
- PMCID: PMC8111137
- DOI: 10.1128/JCM.02342-20
Evaluating 10 Commercially Available SARS-CoV-2 Rapid Serological Tests by Use of the STARD (Standards for Reporting of Diagnostic Accuracy Studies) Method
Abstract
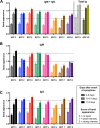
Numerous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) rapid serological tests have been developed, but their accuracy has usually been assessed using very few samples, and rigorous comparisons between these tests are scarce. In this study, we evaluated and compared 10 commercially available SARS-CoV-2 rapid serological tests using the STARD (Standards for Reporting of Diagnostic Accuracy Studies) methodology. Two hundred fifty serum samples from 159 PCR-confirmed SARS-CoV-2 patients (collected 0 to 32 days after the onset of symptoms) were tested with rapid serological tests. Control serum samples (n = 254) were retrieved from pre-coronavirus disease (COVID) periods from patients with other coronavirus infections (n = 11), positivity for rheumatoid factors (n = 3), IgG/IgM hyperglobulinemia (n = 9), malaria (n = 5), or no documented viral infection (n = 226). All samples were tested using rapid lateral flow immunoassays (LFIAs) from 10 manufacturers. Only four tests achieved ≥98% specificity, with the specificities ranging from 75.7% to 99.2%. The sensitivities varied by the day of sample collection after the onset of symptoms, from 31.7% to 55.4% (days 0 to 9), 65.9% to 92.9% (days 10 to 14), and 81.0% to 95.2% (>14 days). Only three of the tests evaluated met French health authorities' thresholds for SARS-CoV-2 serological tests (≥90% sensitivity and ≥98% specificity). Overall, the performances varied greatly between tests, with only one-third meeting acceptable specificity and sensitivity thresholds. Knowledge of the analytical performances of these tests will allow clinicians and, most importantly, laboratorians to use them with more confidence; could help determine the general population's immunological status; and may help diagnose some patients with false-negative real-time reverse transcription-PCR (RT-PCR) results.
Keywords: COVID-19; IgG; IgM; LFIA; RDT; SARS-CoV-2; analytical performances; antibodies; immunoassays; serology.
Copyright © 2021 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

