PAPPA-mediated adipose tissue remodeling mitigates insulin resistance and protects against gestational diabetes in mice and humans
- PMID: 33239385
- PMCID: PMC8375243
- DOI: 10.1126/scitranslmed.aay4145
PAPPA-mediated adipose tissue remodeling mitigates insulin resistance and protects against gestational diabetes in mice and humans
Abstract
Pregnancy is a physiological state of continuous adaptation to changing maternal and fetal nutritional needs, including a reduction of maternal insulin sensitivity allowing for appropriately enhanced glucose availability to the fetus. However, excessive insulin resistance in conjunction with insufficient insulin secretion results in gestational diabetes mellitus (GDM), greatly increasing the risk for pregnancy complications and predisposing both mothers and offspring to future metabolic disease. Here, we report a signaling pathway connecting pregnancy-associated plasma protein A (PAPPA) with adipose tissue expansion in pregnancy. Adipose tissue plays a central role in the regulation of insulin sensitivity, and we show that, in both mice and humans, pregnancy caused remodeling of adipose tissue evidenced by altered adipocyte size, vascularization, and in vitro expansion capacity. PAPPA is known to be a metalloprotease secreted by human placenta that modulates insulin-like growth factor (IGF) bioavailability through prolteolysis of IGF binding proteins (IGFBPs) 2, 4, and 5. We demonstrate that recombinant PAPPA can stimulate ex vivo human adipose tissue expansion in an IGFBP-5- and IGF-1-dependent manner. Moreover, mice lacking PAPPA displayed impaired adipose tissue remodeling, pregnancy-induced insulin resistance, and hepatic steatosis, recapitulating multiple aspects of human GDM. In a cohort of 6361 pregnant women, concentrations of circulating PAPPA are inversely correlated with glycemia and odds of developing GDM. These data identify PAPPA and the IGF signaling pathway as necessary for the regulation of maternal adipose tissue physiology and systemic glucose homeostasis, with consequences for long-term metabolic risk and potential for therapeutic use.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures

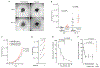
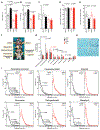
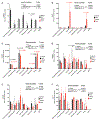

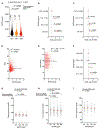
Comment in
-
Adipose tissue remodelling in pregnancy.Nat Rev Endocrinol. 2021 Feb;17(2):67. doi: 10.1038/s41574-020-00461-2. Nat Rev Endocrinol. 2021. PMID: 33328605 No abstract available.
Similar articles
-
Depot-specific and GH-dependent regulation of IGF binding protein-4, pregnancy-associated plasma protein-A, and stanniocalcin-2 in murine adipose tissue.Growth Horm IGF Res. 2018 Apr;39:54-61. doi: 10.1016/j.ghir.2018.01.001. Epub 2018 Feb 3. Growth Horm IGF Res. 2018. PMID: 29398370
-
Human adipose tissue expansion in pregnancy is impaired in gestational diabetes mellitus.Diabetologia. 2015 Sep;58(9):2106-14. doi: 10.1007/s00125-015-3662-0. Epub 2015 Jun 13. Diabetologia. 2015. PMID: 26067361 Free PMC article.
-
Lack of functional pregnancy-associated plasma protein-A (PAPPA) compromises mouse ovarian steroidogenesis and female fertility.Biol Reprod. 2010 Jun;82(6):1129-38. doi: 10.1095/biolreprod.109.079517. Epub 2010 Feb 3. Biol Reprod. 2010. PMID: 20130263 Free PMC article.
-
Gestational Diabetes Mellitus and Energy-Dense Diet: What Is the Role of the Insulin/IGF Axis?Front Endocrinol (Lausanne). 2022 Jun 23;13:916042. doi: 10.3389/fendo.2022.916042. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35813659 Free PMC article. Review.
-
Insulin secretion and insulin resistance in pregnancy and GDM. Implications for diagnosis and management.Diabetes. 1991 Dec;40 Suppl 2:18-24. doi: 10.2337/diab.40.2.s18. Diabetes. 1991. PMID: 1748255 Review.
Cited by
-
Placenta-Derived Exosomes as a Modulator in Maternal Immune Tolerance During Pregnancy.Front Immunol. 2021 May 11;12:671093. doi: 10.3389/fimmu.2021.671093. eCollection 2021. Front Immunol. 2021. PMID: 34046039 Free PMC article. Review.
-
The identification of distinct protective and susceptibility mechanisms for hip osteoarthritis: findings from a genome-wide association study meta-analysis of minimum joint space width and Mendelian randomisation cluster analyses.EBioMedicine. 2023 Sep;95:104759. doi: 10.1016/j.ebiom.2023.104759. Epub 2023 Aug 22. EBioMedicine. 2023. PMID: 37619450 Free PMC article.
-
Insulin-like growth factor binding protein 5: Diverse roles in cancer.Front Oncol. 2022 Nov 17;12:1052457. doi: 10.3389/fonc.2022.1052457. eCollection 2022. Front Oncol. 2022. PMID: 36465383 Free PMC article. Review.
-
Risk prediction models of gestational diabetes mellitus before 16 gestational weeks.BMC Pregnancy Childbirth. 2022 Dec 1;22(1):889. doi: 10.1186/s12884-022-05219-4. BMC Pregnancy Childbirth. 2022. PMID: 36456970 Free PMC article.
-
Associations between first-trimester screening biomarkers and maternal characteristics with gestational diabetes mellitus in Chinese women.Front Endocrinol (Lausanne). 2024 Aug 8;15:1383706. doi: 10.3389/fendo.2024.1383706. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39175575 Free PMC article.
References
-
- Zhang C, Olsen SF, Hinkle SN, Gore-Langton RE, Vaag A, Grunnet LG, Yeung EH, Bao W, Bowers K, Liu A, Mills JL, Sherman S, Gaskins AJ, Ley SH, Madsen CM, Chavarro JE, Hu FB; Diabetes & Women’s Health Study team, Diabetes & Women’s Health (DWH) Study: An observational study of long-term health consequences of gestational diabetes, their determinants and underlying mechanisms in the USA and Denmark. BMJ Open 9, e025517 (2019). - PMC - PubMed
-
- McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P, Gestational diabetes mellitus. Nat. Rev. Dis. Primers 5, 47 (2019). - PubMed
-
- Kjos SL, Peters RK, Xiang A, Henry OA, Montoro M, Buchanan TA, Predicting future diabetes in Latino women with gestational diabetes. Utility of early postpartum glucose tolerance testing. Diabetes 44, 586–591 (1995). - PubMed
-
- Bellamy L, Casas J-P, Hingorani AD, Williams D, Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 373, 1773–1779 (2009). - PubMed
-
- Kwak SH, Choi SH, Kim K, Jung HS, Cho YM, Lim S, Cho NH, Kim SY, Park KS, Jang HC, Prediction of type 2 diabetes in women with a history of gestational diabetes using a genetic risk score. Diabetologia 56, 2556–2563 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

