Systemic cancer therapy with engineered adenovirus that evades innate immunity
- PMID: 33239388
- PMCID: PMC9195642
- DOI: 10.1126/scitranslmed.abc6659
Systemic cancer therapy with engineered adenovirus that evades innate immunity
Abstract
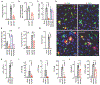
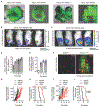
Oncolytic virus therapy is a cancer treatment modality that has the potential to improve outcomes for patients with currently incurable malignancies. Although intravascular delivery of therapeutic viruses provides access to disseminated tumors, this delivery route exposes the virus to opsonizing and inactivating factors in the blood, which limit the effective therapeutic virus dose and contribute to activation of systemic toxicities. When human species C adenovirus HAdv-C5 is delivered intravenously, natural immunoglobulin M (IgM) antibodies and coagulation factor X rapidly opsonize HAdv-C5, leading to virus sequestration in tissue macrophages and promoting infection of liver cells, triggering hepatotoxicity. Here, we showed that natural IgM antibody binds to the hypervariable region 1 (HVR1) of the main HAdv-C5 capsid protein hexon. Using compound targeted mutagenesis of hexon HVR1 loop and other functional sites that mediate virus-host interactions, we engineered and obtained a high-resolution cryo-electron microscopy structure of an adenovirus vector, Ad5-3M, which resisted inactivation by blood factors, avoided sequestration in liver macrophages, and failed to trigger hepatotoxicity after intravenous delivery. Systemic delivery of Ad5-3M to mice with localized or disseminated lung cancer led to viral replication in tumor cells, suppression of tumor growth, and prolonged survival. Thus, compound targeted mutagenesis of functional sites in the virus capsid represents a generalizable approach to tailor virus interactions with the humoral and cellular arms of the immune system, enabling generation of "designer" viruses with improved therapeutic properties.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
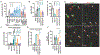



References
-
- Senior M, Checkpoint inhibitors go viral. Nat Biotechnol 37, 12–17 (2019). - PubMed
-
- Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH Jr., Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, Coffin RS, Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 33, 2780–2788 (2015). - PubMed
-
- Andtbacka RH, Ross M, Puzanov I, Milhem M, Collichio F, Delman KA, Amatruda T, Zager JS, Cranmer L, Hsueh E, Chen L, Shilkrut M, Kaufman HL, Patterns of Clinical Response with Talimogene Laherparepvec (T-VEC) in Patients with Melanoma Treated in the OPTiM Phase III Clinical Trial. Annals of surgical oncology 23, 4169–4177 (2016). - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

