Obesity induces gut microbiota alterations and augments acute graft-versus-host disease after allogeneic stem cell transplantation
- PMID: 33239390
- PMCID: PMC8525601
- DOI: 10.1126/scitranslmed.aay7713
Obesity induces gut microbiota alterations and augments acute graft-versus-host disease after allogeneic stem cell transplantation
Abstract
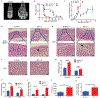
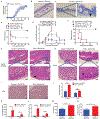
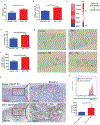
The efficacy of allogeneic hematopoietic stem cell transplantation (allo-HSCT) is limited by acute and chronic graft-versus-host disease (GVHD). The impact of obesity on allo-HSCT outcomes is poorly understood. Here, we report that obesity had a negative and selective impact on acute gut GVHD after allo-HSCT in mice with diet-induced obesity (DIO). These animals exhibited increased gut permeability, endotoxin translocation across the gut, and radiation-induced gastrointestinal damage after allo-HSCT. After allo-HSCT, both male and female DIO mouse recipients showed increased proinflammatory cytokine production and expression of the GVHD marker ST2 (IL-33R) and MHC class II molecules; they also exhibited decreased survival associated with acute severe gut GVHD. This rapid-onset, obesity-associated gut GVHD depended on donor CD4+ T cells and occurred even with a minor MHC mismatch between donor and recipient animals. Retrospective analysis of clinical cohorts receiving allo-HSCT transplants from unrelated donors revealed that recipients with a high body mass index (BMI, >30) had reduced survival and higher serum ST2 concentrations compared with nonobese transplant recipients. Assessment of both DIO mice and allo-HSCT recipients with a high BMI revealed reduced gut microbiota diversity and decreased Clostridiaceae abundance. Prophylactic antibiotic treatment protected DIO mouse recipients from endotoxin translocation across the gut and increased inflammatory cytokine production, as well as gut pathology and mortality, but did not protect against later development of chronic skin GVHD. These results suggest that obesity-induced alterations of the gut microbiota may affect GVHD after allo-HSCT in DIO mice, which could be ameliorated by prophylactic antibiotic treatment.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures




References
-
- Flegal KM, Carroll MD, Kit BK, Ogden CL, Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307, 491–497 (2012). - PubMed
-
- Navarro WH et al. , Effect of body mass index on mortality of patients with lymphoma undergoing autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant 12, 541–551 (2006). - PubMed
-
- Fuji S et al. , Possible association between obesity and posttransplantation complications including infectious diseases and acute graft-versus-host disease. Biol Blood Marrow Transplant 15, 73–82 (2009). - PubMed
-
- Jaime-Perez JC et al. , Obesity is associated with higher overall survival in patients undergoing an outpatient reduced-intensity conditioning hematopoietic stem cell transplant. Blood Cells Mol Dis 51, 61–65 (2013). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

