Esaxerenone (CS-3150) in Patients with Type 2 Diabetes and Microalbuminuria (ESAX-DN): Phase 3 Randomized Controlled Clinical Trial
- PMID: 33239409
- PMCID: PMC7769030
- DOI: 10.2215/CJN.06870520
Esaxerenone (CS-3150) in Patients with Type 2 Diabetes and Microalbuminuria (ESAX-DN): Phase 3 Randomized Controlled Clinical Trial
Abstract
Background and objectives: Diabetic kidney disease is an important complication of type 2 diabetes. In a phase 2b study, adding esaxerenone to renin-angiotensin system inhibitors dose dependently reduced the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and microalbuminuria. This 52-week phase 3 study further investigated the effects of esaxerenone on the urinary albumin-to-creatinine ratio in this patient group.
Design, setting, participants, & measurements: In this multicenter, randomized, double-blind study, patients with type 2 diabetes and a urinary albumin-to-creatinine ratio of 45 to <300 mg/g creatinine treated with renin-angiotensin system inhibitors were randomized to esaxerenone or placebo for 52 weeks (n=455). Esaxerenone was initiated at 1.25 mg/d and titrated to 2.5 mg/d on the basis of serum potassium monitoring. The primary endpoint was the proportion of patients achieving urinary albumin-to-creatinine ratio remission (<30 mg/g creatinine and ≥30% reduction from baseline on two consecutive occasions).
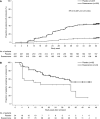
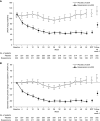
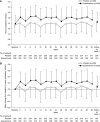
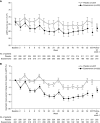
Results: Overall, 49 (22%) and nine (4%) patients in the esaxerenone and placebo groups, respectively, achieved urinary albumin-to-creatinine ratio remission (absolute difference 18%; 95% confidence interval, 12% to 25%; P<0.001). The percent change in urinary albumin-to-creatinine ratio from baseline to end of treatment was significantly higher with esaxerenone versus placebo (-58% versus 8%; geometric least-squares mean ratio to placebo 0.38, 95% confidence interval, 0.33 to 0.44). There was a significant improvement with esaxerenone versus placebo in time to first remission (hazard ratio, 5.13; 95% confidence interval, 3.27 to 8.04) and time to first transition to urinary albumin-to-creatinine ratio ≥300 mg/g creatinine (hazard ratio, 0.23; 95% confidence interval, 0.11 to 0.48). More patients had a serum potassium level ≥6.0 or ≥5.5 mEq/L on two consecutive measurements in the esaxerenone group (20 [9%]) versus placebo (5 [2%]); these events were asymptomatic and resolved after dosage reduction or treatment discontinuation.
Conclusions: Adding esaxerenone to existing renin-angiotensin system inhibitor therapy in patients with type 2 diabetes and microalbuminuria increased the likelihood of albuminuria returning to normal levels, and reduced progression of albuminuria to higher levels.
Keywords: diabetes mellitus; esaxerenone; microalbuminuria.
Copyright © 2020 by the American Society of Nephrology.
Figures

Comment in
-
Mineralocorticoid Receptor Antagonists for Diabetic Kidney Disease.Clin J Am Soc Nephrol. 2020 Dec 7;15(12):1696-1698. doi: 10.2215/CJN.16201020. Epub 2020 Nov 25. Clin J Am Soc Nephrol. 2020. PMID: 33239412 Free PMC article. No abstract available.
References
-
- Lin YC, Chang YH, Yang SY, Wu KD, Chu TS: Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc 117: 662–675, 2018 - PubMed
-
- Norris KC, Smoyer KE, Rolland C, Van der Vaart J, Grubb EB: Albuminuria, serum creatinine, and estimated glomerular filtration rate as predictors of cardio-renal outcomes in patients with type 2 diabetes mellitus and kidney disease: A systematic literature review. BMC Nephrol 19: 36, 2018 - PMC - PubMed
-
- Vupputuri S, Kimes TM, Calloway MO, Christian JB, Bruhn D, Martin AA, Nichols GA: The economic burden of progressive chronic kidney disease among patients with type 2 diabetes. J Diabetes Complications 28: 10–16, 2014 - PubMed
-
- Dinneen SF, Gerstein HC: The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med 157: 1413–1418, 1997 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

