A Whole Blood Enzyme-Linked Immunospot Assay for Functional Immune Endotyping of Septic Patients
- PMID: 33239423
- PMCID: PMC8026772
- DOI: 10.4049/jimmunol.2001088
A Whole Blood Enzyme-Linked Immunospot Assay for Functional Immune Endotyping of Septic Patients
Abstract
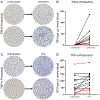
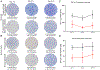
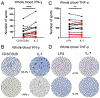
Sepsis initiates simultaneous pro- and anti-inflammatory processes, the pattern and intensity of which vary over time. The inability to evaluate the immune status of patients with sepsis in a rapid and quantifiable manner has undoubtedly been a major reason for the failure of many therapeutic trials. Although there has been considerable effort to immunophenotype septic patients, these methods have often not accurately assessed the functional state of host immunity, lack dynamic range, and are more reflective of molecular processes rather than host immunity. In contrast, ELISpot assay measures the number and intensity of cytokine-secreting cells and has excellent dynamic range with rapid turnaround. We investigated the ability of a (to our knowledge) novel whole blood ELISpot assay and compared it with a more traditional ELISpot assay using PBMCs in sepsis. IFN-γ and TNF-α ELISpot assays on whole blood and PBMCs were undertaken in control, critically ill nonseptic, and septic patients. Whole blood ELISpot was easy to perform, and results were generally comparable to PBMC-based ELISpot. However, the whole blood ELISpot assay revealed that nonmonocyte, myeloid populations are a significant source of ex vivo TNF-α production. Septic patients who died had early, profound, and sustained suppression of innate and adaptive immunity. A cohort of septic patients had increased cytokine production compared with controls consistent with either an appropriate or excessive immune response. IL-7 restored ex vivo IFN-γ production in septic patients. The whole blood ELISpot assay offers a significant advance in the ability to immunophenotype patients with sepsis and to guide potential new immunotherapies.
Copyright © 2020 by The American Association of Immunologists, Inc.
Conflict of interest statement
Disclosures
Patent pending for intellectual property for whole blood ELISpot immune phenotyping for R.S.H., M.B.M., C.C.C., I.R.T., K.E.R. The other authors have no financial conflicts of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

