Subdominance in Antibody Responses: Implications for Vaccine Development
- PMID: 33239435
- PMCID: PMC7709521
- DOI: 10.1128/MMBR.00078-20
Subdominance in Antibody Responses: Implications for Vaccine Development
Abstract
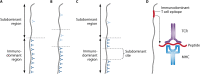
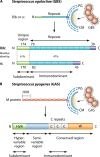
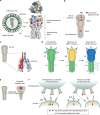
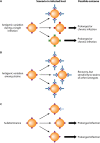
Vaccines work primarily by eliciting antibodies, even when recovery from natural infection depends on cellular immunity. Large efforts have therefore been made to identify microbial antigens that elicit protective antibodies, but these endeavors have encountered major difficulties, as witnessed by the lack of vaccines against many pathogens. This review summarizes accumulating evidence that subdominant protein regions, i.e., surface-exposed regions that elicit relatively weak antibody responses, are of particular interest for vaccine development. This concept may seem counterintuitive, but subdominance may represent an immune evasion mechanism, implying that the corresponding region potentially is a key target for protective immunity. Following a presentation of the concepts of immunodominance and subdominance, the review will present work on subdominant regions in several major human pathogens: the protozoan Plasmodium falciparum, two species of pathogenic streptococci, and the dengue and influenza viruses. Later sections are devoted to the molecular basis of subdominance, its potential role in immune evasion, and general implications for vaccine development. Special emphasis will be placed on the fact that a whole surface-exposed protein domain can be subdominant, as demonstrated for all of the pathogens described here. Overall, the available data indicate that subdominant protein regions are of much interest for vaccine development, not least in bacterial and protozoal systems, for which antibody subdominance remains largely unexplored.
Keywords: Plasmodium falciparum; Streptococcus agalactiae; Streptococcus pyogenes; antibodies; dengue virus; immune escape; immunodominance; influenza virus; subdominance; vaccine.
Copyright © 2020 American Society for Microbiology.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

