Tolerogenic nanoparticles suppress central nervous system inflammation
- PMID: 33239445
- PMCID: PMC7749362
- DOI: 10.1073/pnas.2016451117
Tolerogenic nanoparticles suppress central nervous system inflammation
Abstract
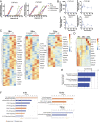
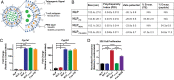
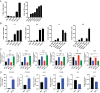
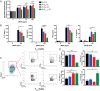
Therapeutic approaches for the induction of immune tolerance remain an unmet clinical need for the treatment of autoimmune diseases, including multiple sclerosis (MS). Based on its role in the control of the immune response, the ligand-activated transcription factor aryl hydrocarbon receptor (AhR) is a candidate target for novel immunotherapies. Here, we report the development of AhR-activating nanoliposomes (NLPs) to induce antigen-specific tolerance. NLPs loaded with the AhR agonist ITE and a T cell epitope from myelin oligodendrocyte glycoprotein (MOG)35-55 induced tolerogenic dendritic cells and suppressed the development of experimental autoimmune encephalomyelitis (EAE), a preclinical model of MS, in preventive and therapeutic setups. EAE suppression was associated with the expansion of MOG35-55-specific FoxP3+ regulatory T cells (Treg cells) and type 1 regulatory T cells (Tr1 cells), concomitant with a reduction in central nervous system-infiltrating effector T cells (Teff cells). Notably, NLPs induced bystander suppression in the EAE model established in C57BL/6 × SJL F1 mice. Moreover, NLPs ameliorated chronic progressive EAE in nonobese diabetic mice, a model which resembles some aspects of secondary progressive MS. In summary, these studies describe a platform for the therapeutic induction of antigen-specific tolerance in autoimmune diseases.
Keywords: EAE; MS; antigen-specific therapy; autoimmunity; nanoparticles.
Conflict of interest statement
Competing interest statement: J.E.K, A.J., N.K., S.T., D.N., A.P., V.P.S. are/were employees at AnTolRx; F.J.Q. is a consultant at AnTolRx. This work was partially funded by AnTolRx.
Figures




References
-
- Serra P., Santamaria P., Antigen-specific therapeutic approaches for autoimmunity. Nat. Biotechnol. 37, 238–251 (2019). - PubMed
-
- Klein L., Robey E. A., Hsieh C. S., Central CD4+ T cell tolerance: deletion versus regulatory T cell differentiation. Nat. Rev. Immunol. 19, 7–18 (2019). - PubMed
-
- Roncarolo M. G., Gregori S., Bacchetta R., Battaglia M., Gagliani N., The biology of T regulatory type 1 cells and their therapeutic application in immune-mediated diseases. Immunity 49, 1004–1019 (2018). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

