KRAB zinc finger protein diversification drives mammalian interindividual methylation variability
- PMID: 33239447
- PMCID: PMC7733849
- DOI: 10.1073/pnas.2017053117
KRAB zinc finger protein diversification drives mammalian interindividual methylation variability
Abstract
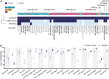
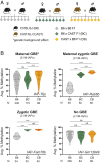
Most transposable elements (TEs) in the mouse genome are heavily modified by DNA methylation and repressive histone modifications. However, a subset of TEs exhibit variable methylation levels in genetically identical individuals, and this is associated with epigenetically conferred phenotypic differences, environmental adaptability, and transgenerational epigenetic inheritance. The evolutionary origins and molecular mechanisms underlying interindividual epigenetic variability remain unknown. Using a repertoire of murine variably methylated intracisternal A-particle (VM-IAP) epialleles as a model, we demonstrate that variable DNA methylation states at TEs are highly susceptible to genetic background effects. Taking a classical genetics approach coupled with genome-wide analysis, we harness these effects and identify a cluster of KRAB zinc finger protein (KZFP) genes that modifies VM-IAPs in trans in a sequence-specific manner. Deletion of the cluster results in decreased DNA methylation levels and altered histone modifications at the targeted VM-IAPs. In some cases, these effects are accompanied by dysregulation of neighboring genes. We find that VM-IAPs cluster together phylogenetically and that this is linked to differential KZFP binding, suggestive of an ongoing evolutionary arms race between TEs and this large family of epigenetic regulators. These findings indicate that KZFP divergence and concomitant evolution of DNA binding capabilities are mechanistically linked to methylation variability in mammals, with implications for phenotypic variation and putative paradigms of mammalian epigenetic inheritance.
Keywords: DNA methylation; KRAB zinc finger proteins; VM-IAP; endogenous retrovirus; metastable epiallele.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures





References
-
- Sapienza C., Paquette J., Tran T. H., Peterson A., Epigenetic and genetic factors affect transgene methylation imprinting. Development 107, 165–168 (1989). - PubMed
-
- Allen N. D., Norris M. L., Surani M. A., Epigenetic control of transgene expression and imprinting by genotype-specific modifiers. Cell 61, 853–861 (1990). - PubMed
-
- Engler P., et al. , A strain-specific modifier on mouse chromosome 4 controls the methylation of independent transgene loci. Cell 65, 939–947 (1991). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

