Structure and dsRNA-binding activity of the Birnavirus Drosophila X Virus VP3 protein
- PMID: 33239452
- PMCID: PMC7851550
- DOI: 10.1128/JVI.02166-20
Structure and dsRNA-binding activity of the Birnavirus Drosophila X Virus VP3 protein
Abstract
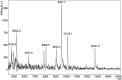
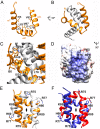
The Birnavirus multifunctional protein VP3 plays an essential role coordinating the virus life cycle, interacting with the capsid protein VP2, with the RNA-dependent RNA polymerase VP1 and with the dsRNA genome. Furthermore, the role of this protein in controlling host cell responses triggered by dsRNA and preventing gene silencing has been recently demonstrated. Here we report the X-ray structure and dsRNA-binding activity of the N-terminal domain of Drosophila X virus (DXV) VP3. The domain folds in a bundle of three α-helices and arranges as a dimer, exposing to the surface a well-defined cluster of basic residues. Site directed mutagenesis combined with Electrophoretic Mobility Shift Assays (EMSA) and Surface Plasmon Resonance (SPR) revealed that this cluster, as well as a flexible and positively charged region linking the first and second globular domains of DXV VP3, are essential for dsRNA-binding. Also, RNA silencing studies performed in insect cell cultures confirmed the crucial role of this VP3 domain for the silencing suppression activity of the protein.IMPORTANCE The Birnavirus moonlighting protein VP3 plays crucial roles interacting with the dsRNA genome segments to form stable ribonucleoprotein complexes and controlling host cell immune responses, presumably by binding to and shielding the dsRNA from recognition by the host silencing machinery. The structural, biophysical and functional data presented in this work has identified the N-terminal domain of VP3 as responsible for the dsRNA-binding and silencing suppression activities of the protein in Drosophila X virus.
Copyright © 2020 American Society for Microbiology.
Figures






References
-
- Delmas B, Mundt E, Vakharia VN, Wu JL. 2011. Birnaviridae, p 499–507. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus taxonomy: classification and nomenclature of viruses. Ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA.
LinkOut - more resources
Full Text Sources
Other Literature Sources

