Protective effects of taxifolin on pazopanib-induced liver toxicity: an experimental rat model
- PMID: 33239495
- PMCID: PMC8150244
- DOI: 10.1538/expanim.20-0103
Protective effects of taxifolin on pazopanib-induced liver toxicity: an experimental rat model
Abstract
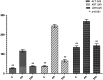
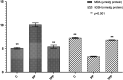
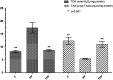
Pazopanib is a tyrosine kinase inhibitor that is generally used for the treatment of metastatic renal cell cancer and advanced soft tissue sarcoma. It can cause various degrees of hepatotoxicity. Our study aimed to investigate the effect of taxifolin on pazopanib-induced liver toxicity. A total of 18 rats were divided into three groups: the pazopanib (PP), pazopanib plus taxifolin (TPP), and control (C) group. Taxifolin was administered to the TPP (n=6) group with a dose of 50 mg/kg. Distilled water was orally admnistered to the C (n=6) and PP (n=6) groups as a solvent. Subsequently, pazopanib 200 mg/kg was administered to the TPP and PP groups via the stomach. This procedure was repeated once a day for four weeks. Then, all rats were sacrificed, and their livers were removed. Malondialdehyde (MDA), total glutathione (tGSH), total oxidant status (TOS), and total antioxidant status (TAS) levels were evaluated. MDA and TOS levels were higher in the PP group compared with the levels of the other parameters (P<0.001). tGSH and TAS levels were lower in the PP group than in the TPP and C groups (P<0.001), and the aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) levels were higher. Furthermore, liver tissue damage, including hemorrhage, hydropic degeneration, and necrosis was observed in the PP group. Administration of taxifolin before pazopanib significantly improved degenerative changes. Our study demonstrated that the administration of taxifolin is significantly effective in preventing pazopanib-induced hepatotoxicity in rats.
Keywords: experimental models; liver toxicity; oxidative stress; pazopanib; taxifolin.
Figures


References
-
- Karch A, Koch A, Grünwald V. A phase II trial comparing pazopanib with doxorubicin as first-line treatment in elderly patients with metastatic or advanced soft tissue sarcoma (EPAZ): study protocol for a randomized controlled trial. Trials. 2016; 17: 312. doi: 10.1186/s13063-016-1434-x - DOI - PMC - PubMed
-
- Dinkic C, Eichbaum M, Schmidt M, Grischke EM, Gebauer G, Fricke HC, et al. Pazopanib (GW786034) and cyclophosphamide in patients with platinum-resistant, recurrent, pre-treated ovarian cancer - Results of the PACOVAR-trial. Gynecol Oncol. 2017; 146: 279–284. doi: 10.1016/j.ygyno.2017.05.013 - DOI - PubMed
-
- Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, Schöffski P, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol. 2009; 27: 3126–3132. doi: 10.1200/JCO.2008.21.3223 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

