Remote near infrared identification of pathogens with multiplexed nanosensors
- PMID: 33239609
- PMCID: PMC7689463
- DOI: 10.1038/s41467-020-19718-5
Remote near infrared identification of pathogens with multiplexed nanosensors
Abstract
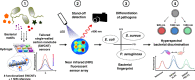
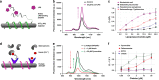
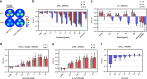
Infectious diseases are worldwide a major cause of morbidity and mortality. Fast and specific detection of pathogens such as bacteria is needed to combat these diseases. Optimal methods would be non-invasive and without extensive sample-taking/processing. Here, we developed a set of near infrared (NIR) fluorescent nanosensors and used them for remote fingerprinting of clinically important bacteria. The nanosensors are based on single-walled carbon nanotubes (SWCNTs) that fluoresce in the NIR optical tissue transparency window, which offers ultra-low background and high tissue penetration. They are chemically tailored to detect released metabolites as well as specific virulence factors (lipopolysaccharides, siderophores, DNases, proteases) and integrated into functional hydrogel arrays with 9 different sensors. These hydrogels are exposed to clinical isolates of 6 important bacteria (Staphylococcus aureus, Escherichia coli,…) and remote (≥25 cm) NIR imaging allows to identify and distinguish bacteria. Sensors are also spectrally encoded (900 nm, 1000 nm, 1250 nm) to differentiate the two major pathogens P. aeruginosa as well as S. aureus and penetrate tissue (>5 mm). This type of multiplexing with NIR fluorescent nanosensors enables remote detection and differentiation of important pathogens and the potential for smart surfaces.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Willyard C. Drug-resistant bacteria ranked. Nature. 2017;543:15. - PubMed
-
- Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392:75–87. - PubMed
-
- Shohat N, et al. Hip and knee section, what is the definition of a periprosthetic joint infection (PJI) of the knee and the hip? Can the same criteria be used for both joints?: Proceedings of international consensus on orthopedic infections. J. Arthroplast. 2019;34:325–327. - PubMed
-
- Váradi L, et al. Methods for the detection and identification of pathogenic bacteria: past, present, and future. Chem. Soc. Rev. 2017;46:4818–4832. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

