Differences in visually induced MEG oscillations reflect differences in deep cortical layer activity
- PMID: 33239652
- PMCID: PMC7688644
- DOI: 10.1038/s42003-020-01438-7
Differences in visually induced MEG oscillations reflect differences in deep cortical layer activity
Abstract
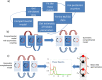
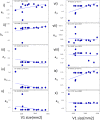
Neural activity is organized at multiple scales, ranging from the cellular to the whole brain level. Connecting neural dynamics at different scales is important for understanding brain pathology. Neurological diseases and disorders arise from interactions between factors that are expressed in multiple scales. Here, we suggest a new way to link microscopic and macroscopic dynamics through combinations of computational models. This exploits results from statistical decision theory and Bayesian inference. To validate our approach, we used two independent MEG datasets. In both, we found that variability in visually induced oscillations recorded from different people in simple visual perception tasks resulted from differences in the level of inhibition specific to deep cortical layers. This suggests differences in feedback to sensory areas and each subject's hypotheses about sensations due to differences in their prior experience. Our approach provides a new link between non-invasive brain imaging data, laminar dynamics and top-down control.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

