The fibrotic and immune microenvironments as targetable drivers of metastasis
- PMID: 33239677
- PMCID: PMC7782519
- DOI: 10.1038/s41416-020-01172-1
The fibrotic and immune microenvironments as targetable drivers of metastasis
Abstract
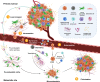
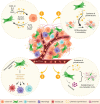
Although substantial progress has been made over the past 40 years in treating patients with cancer, effective therapies for those who are diagnosed with advanced metastatic disease are still few and far between. Cancer cells do not exist in isolation: rather, they exist within a complex microenvironment composed of stromal cells and extracellular matrix. Within this tumour microenvironment exists an interplay between the two main stromal cell subtypes, cancer-associated fibroblasts (CAFs) and immune cells, that are important in controlling metastasis. A complex network of paracrine signalling pathways between CAFs, immune cells and tumour cells are involved at multiple stages of the metastatic process, from invasion and intravasation at the primary tumour site to extravasation and colonisation in the metastatic site. Heterogeneity and plasticity within stromal cell populations also contribute to the complexity. Although many of these processes are likely to be common to a number of metastatic sites, we will describe in detail the interplay within the liver, a preferred site of metastasis for many tumours. A greater understanding of these networks provides opportunities for the design of new therapeutic approaches for targeting the metastatic disease.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

