The lingering mysteries of metastatic recurrence in breast cancer
- PMID: 33239679
- PMCID: PMC7782773
- DOI: 10.1038/s41416-020-01161-4
The lingering mysteries of metastatic recurrence in breast cancer
Abstract
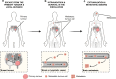
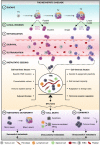
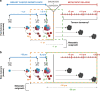
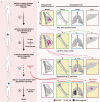
Despite being the hallmark of cancer that is responsible for the highest number of deaths, very little is known about the biology of metastasis. Metastatic disease typically manifests after a protracted period of undetectable disease following surgery or systemic therapy, owing to relapse or recurrence. In the case of breast cancer, metastatic relapse can occur months to decades after initial diagnosis and treatment. In this review, we provide an overview of the known key factors that influence metastatic recurrence, with the goal of highlighting the critical unanswered questions that still need to be addressed to make a difference in the mortality of breast cancer patients.
Conflict of interest statement
The authors declare no competing interests.
Figures
Comment in
-
Comment on "The lingering mysteries of metastatic recurrence in breast cancer".Br J Cancer. 2023 Feb;128(3):484-485. doi: 10.1038/s41416-022-02012-0. Epub 2022 Oct 31. Br J Cancer. 2023. PMID: 36316559 Free PMC article. No abstract available.
References
-
- Ferlay, J., Ervik, M., Lam, F., Colombet, M., Mery, L., Piñeros, M. et al. Global Cancer Observatory: Cancer Tomorrow. https://gco.iarc.fr/tomorrow (International Agency for Research on Cancer, Lyon, France, 2018).
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. - PubMed
-
- Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat. Rev. Cancer. 2004;4:448–456. - PubMed
-
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

