The barley pan-genome reveals the hidden legacy of mutation breeding
- PMID: 33239781
- PMCID: PMC7759462
- DOI: 10.1038/s41586-020-2947-8
The barley pan-genome reveals the hidden legacy of mutation breeding
Abstract
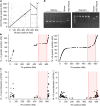
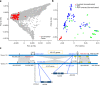
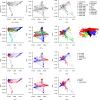
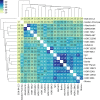
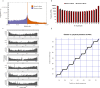
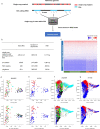
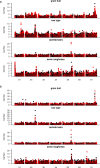
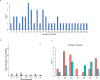
Genetic diversity is key to crop improvement. Owing to pervasive genomic structural variation, a single reference genome assembly cannot capture the full complement of sequence diversity of a crop species (known as the 'pan-genome'1). Multiple high-quality sequence assemblies are an indispensable component of a pan-genome infrastructure. Barley (Hordeum vulgare L.) is an important cereal crop with a long history of cultivation that is adapted to a wide range of agro-climatic conditions2. Here we report the construction of chromosome-scale sequence assemblies for the genotypes of 20 varieties of barley-comprising landraces, cultivars and a wild barley-that were selected as representatives of global barley diversity. We catalogued genomic presence/absence variants and explored the use of structural variants for quantitative genetic analysis through whole-genome shotgun sequencing of 300 gene bank accessions. We discovered abundant large inversion polymorphisms and analysed in detail two inversions that are frequently found in current elite barley germplasm; one is probably the product of mutation breeding and the other is tightly linked to a locus that is involved in the expansion of geographical range. This first-generation barley pan-genome makes previously hidden genetic variation accessible to genetic studies and breeding.
Conflict of interest statement
The authors declare no competing interests.
Figures





Comment in
-
Insights on decoding wheat and barley genomes.Funct Integr Genomics. 2021 Mar;21(2):157-159. doi: 10.1007/s10142-021-00774-z. Epub 2021 Feb 17. Funct Integr Genomics. 2021. PMID: 33598867 No abstract available.
References
-
- Bayer PE, Golicz AA, Scheben A, Batley J, Edwards D. Plant pan-genomes are the new reference. Nat. Plants. 2020;6:914–920. - PubMed
-
- Dawson IK, et al. Barley: a translational model for adaptation to climate change. New Phytol. 2015;206:913–931. - PubMed
-
- Stein, N. & Muehlbauer, G. J. The Barley Genome (Springer, 2018).
-
- International Barley Genome Sequencing Consortium A physical, genetic and functional sequence assembly of the barley genome. Nature. 2012;491:711–716. - PubMed
-
- Mascher M, et al. A chromosome conformation capture ordered sequence of the barley genome. Nature. 2017;544:427–433. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

