A map of cis-regulatory elements and 3D genome structures in zebrafish
- PMID: 33239788
- PMCID: PMC8183574
- DOI: 10.1038/s41586-020-2962-9
A map of cis-regulatory elements and 3D genome structures in zebrafish
Abstract
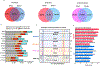
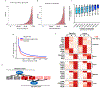
The zebrafish (Danio rerio) has been widely used in the study of human disease and development, and about 70% of the protein-coding genes are conserved between the two species1. However, studies in zebrafish remain constrained by the sparse annotation of functional control elements in the zebrafish genome. Here we performed RNA sequencing, assay for transposase-accessible chromatin using sequencing (ATAC-seq), chromatin immunoprecipitation with sequencing, whole-genome bisulfite sequencing, and chromosome conformation capture (Hi-C) experiments in up to eleven adult and two embryonic tissues to generate a comprehensive map of transcriptomes, cis-regulatory elements, heterochromatin, methylomes and 3D genome organization in the zebrafish Tübingen reference strain. A comparison of zebrafish, human and mouse regulatory elements enabled the identification of both evolutionarily conserved and species-specific regulatory sequences and networks. We observed enrichment of evolutionary breakpoints at topologically associating domain boundaries, which were correlated with strong histone H3 lysine 4 trimethylation (H3K4me3) and CCCTC-binding factor (CTCF) signals. We performed single-cell ATAC-seq in zebrafish brain, which delineated 25 different clusters of cell types. By combining long-read DNA sequencing and Hi-C, we assembled the sex-determining chromosome 4 de novo. Overall, our work provides an additional epigenomic anchor for the functional annotation of vertebrate genomes and the study of evolutionarily conserved elements of 3D genome organization.
Conflict of interest statement
Declaration of interests
The authors declare no competing financial interests.
Figures















References
-
- Gerhard GS et al. Life spans and senescent phenotypes in two strains of Zebrafish (Danio rerio). Experimental gerontology 37, 1055–1068 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

