The Pervasive Effects of ER Stress on a Typical Endocrine Cell: Dedifferentiation, Mesenchymal Shift and Antioxidant Response in the Thyrocyte
- PMID: 33240221
- PMCID: PMC7680880
- DOI: 10.3389/fendo.2020.588685
The Pervasive Effects of ER Stress on a Typical Endocrine Cell: Dedifferentiation, Mesenchymal Shift and Antioxidant Response in the Thyrocyte
Abstract
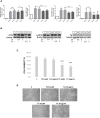
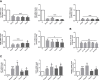
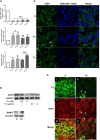
The endoplasmic reticulum stress and the unfolded protein response are triggered following an imbalance between protein load and protein folding. Until recently, two possible outcomes of the unfolded protein response have been considered: life or death. We sought to substantiate a third alternative, dedifferentiation, mesenchymal shift, and activation of the antioxidant response by using typical endocrine cells, i.e. thyroid cells. The thyroid is a unique system both of endoplasmic reticulum stress (a single protein, thyroglobulin represents the majority of proteins synthesized in the endoplasmic reticulum by the thyrocyte) and of polarized epithelium (the single layer of thyrocytes delimiting the follicle). Following endoplasmic reticulum stress, in thyroid cells the folding of thyroglobulin was disrupted. The mRNAs of unfolded protein response were induced or spliced (X-box binding protein-1). Differentiation was inhibited: mRNA levels of thyroid specific genes, and of thyroid transcription factors were dramatically downregulated, at least in part, transcriptionally. The dedifferentiating response was accompanied by an upregulation of mRNAs of antioxidant genes. Moreover, cadherin-1, and the thyroid (and kidney)-specific cadherin-16 mRNAs were downregulated, vimentin, and SNAI1 mRNAs were upregulated. In addition, loss of cortical actin and stress fibers formation were observed. Together, these data indicate that ER stress in thyroid cells induces dedifferentiation, loss of epithelial organization, shift towards a mesenchymal phenotype, and activation of the antioxidant response, highlighting, at the same time, a new and wide strategy to achieve survival following ER stress, and, as a sort of the other side of the coin, a possible new molecular mechanism of decline/loss of function leading to a deficit of thyroid hormones formation.
Keywords: ER stress; antioxidant response; dedifferentiation; mesenchymal phenotype; thyroid.
Copyright © 2020 Ulianich, Mirra, Garbi, Calì, Conza, Treglia, Miraglia, Punzi, Miele, Raciti, Beguinot, Consiglio and Di Jeso.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

