Short stature and SHOX (Short stature homeobox) variants-efficacy of screening using various strategies
- PMID: 33240610
- PMCID: PMC7678493
- DOI: 10.7717/peerj.10236
Short stature and SHOX (Short stature homeobox) variants-efficacy of screening using various strategies
Abstract
Background: SHOX mutations have previously been described as causes of Léri-Weill dyschondrosteosis (LWD), Langer mesomelic dysplasia (LMD), and idiopathic short stature. The loss of X chromosome-Turner syndrome or mosaic 45,X/46,XX or 46,XY-also leads to the heterozygous loss of SHOX in patients with short stature only or with features similar to LWD. The aim of this study was to assess the efficacy of the targeted screening for SHOX variants, which involved different methods in the laboratory analysis of short stature. We determined the significance and positive predictive value of short stature for the detection of SHOX variants.
Methods: Targeted screening for variants in SHOX involving MLPA, sequencing, karyotyping and FISH was performed in the short stature cohort (N = 174) and control cohort (N = 91). The significance of short stature and particular characteristics for the detection of SHOX variants was determined by Fisher's exact test, and the probability of SHOX mutation occurrence was calculated using a forward/stepwise logistic regression model.
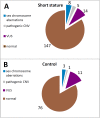
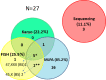
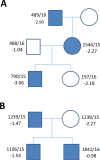
Results: In total, 27 and 15 variants influencing SHOX were detected in the short stature and control cohorts, respectively (p > 0.01). Sex chromosome aberrations and pathogenic CNV resulting in diagnosis were detected in eight (4.6%) and five (2.9%) patients of the short stature group and three (3.3%) and one (1.1%) individuals of the control group. VUS variants were discovered in 14 (8.0%) and 11 (12.1%) individuals of the short stature and control groups, respectively. MLPA demonstrated the detection rate of 13.22%, and it can be used as a frontline method for detection of aberrations involving SHOX. However, only mosaicism of monosomy X with a higher frequency of monosomic cells could be reliably discovered by this method. Karyotyping and FISH can compensate for this limitation; their detection rates in short stature group were 3.55% and 13.46% (N = 52), respectively. FISH proved to be more effective than karyotyping in the study as it could reveal cryptic mosaics in some cases where karyotyping initially failed to detect such a clone. We suggest adding FISH on different tissue than peripheral blood to verify sex-chromosome constitution, especially in cases with karyotypes: 45,X; mosaic 45,X/46,XX or 46,XY; 46,Xidic(Y) detected from blood; in children, where mosaic 45,X was detected prenatally but was not confirmed from peripheral blood. The correlation of short stature with the occurrence of SHOX mutations was insignificant and short stature demonstrates a low positive predictive value-15.5% as unique indicator for SHOX mutations. The typical skeletal signs of LWD, including Madelung deformity and disproportionate growth, positively correlate with the findings of pathogenic SHOX variants (p < 0.01) by Fisher's exact test but not with the findings of VUS variants in SHOX which are more prevalent in the individuals with idiopathic short stature or in the individuals with normal height.
Keywords: FISH; Idiopathic short stature; Karyotyping; Leri-Weill dyschondrosteosis; MLPA; SHOX; Screening for mutations; Sequencing; Short stature; Turner syndrome.
©2020 Capkova et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures

References
-
- Amin N, Mushtaq T, Alvi S. Fifteen-minute consultation: the child with short stature. Archives of Disease in Childhood—Education and Practice. 2015;100:180–184. - PubMed
-
- Benito-Sanz S, Aza-Carmona M, Magano LF, Lapunzina P, Argente J, Campos-Barros A, Heath KE. PAR1 deletions downstream of SHOX are the most frequent defect in a Spanish cohort of Léri-Weill dyschondrosteosis (LWD) probands. Human Mutation. 2006;27(10):1062–1068. doi: 10.1002/humu.9456. - DOI - PubMed
-
- Benito-Sanz S, Barroso E, Heine-Suñer D, Hisado-Oliva A, Romanelli V, Rosell J, Aragones A, Caimari M, Argente J, Ross JL, Zinn AR, Gracia R, Lapunzina P, Campos-Barros A, Heath KE. Clinical and molecular evaluation of SHOX/PAR1 duplications in Leri-Weill dyschondrosteosis (LWD) and idiopathic short stature (ISS) The Journal of Clinical Endocrinology and Metabolism. 2011;96(2):E404-12. doi: 10.1210/jc.2010-1689. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

