Cardiac Adverse Events With Remdesivir in COVID-19 Infection
- PMID: 33240723
- PMCID: PMC7682945
- DOI: 10.7759/cureus.11132
Cardiac Adverse Events With Remdesivir in COVID-19 Infection
Abstract
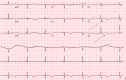
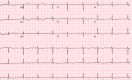
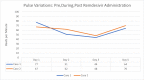
Since December 2019, coronavirus has gradually progressed to a pandemic with no efficacious treatment. Remdesivir is an antiviral medication and inhibitor of viral RNA dependent RNA polymerase with inhibitory action against SARS-CoV virus. Two patients diagnosed with coronavirus infection with worsening respiratory status were initiated with multimodality therapy with antibiotics, steroids and remdesivir. After initiation of remdesivir, the patients' developed bradycardia, with one of the two also showing signs of worsening QT interval. This reverted upon stopping remdesvir therapy. The prevalence of bradycardia with prolonged QT interval is not well-known yet with this medication.
Keywords: bradycardia; qtc prolongation; remdesivir.
Copyright © 2020, Gupta et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
