Hydrogels for 3D Neural Tissue Models: Understanding Cell-Material Interactions at a Molecular Level
- PMID: 33240868
- PMCID: PMC7677185
- DOI: 10.3389/fbioe.2020.601704
Hydrogels for 3D Neural Tissue Models: Understanding Cell-Material Interactions at a Molecular Level
Abstract
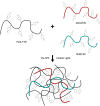
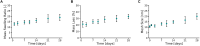
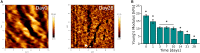
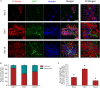
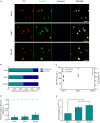
The development of 3D neural tissue analogs is of great interest to a range of biomedical engineering applications including tissue engineering of neural interfaces, treatment of neurodegenerative diseases and in vitro assessment of cell-material interactions. Despite continued efforts to develop synthetic or biosynthetic hydrogels which promote the development of complex neural networks in 3D, successful long-term 3D approaches have been restricted to the use of biologically derived constructs. In this study a poly (vinyl alcohol) biosynthetic hydrogel functionalized with gelatin and sericin (PVA-SG), was used to understand the interplay between cell-cell communication and cell-material interaction. This was used to probe critical short-term interactions that determine the success or failure of neural network growth and ultimately the development of a useful model. Complex primary ventral mesencephalic (VM) neural cells were encapsulated in PVA-SG hydrogels and critical molecular cues that demonstrate mechanosensory interaction were examined. Neuronal presence was constant over the 10 day culture, but the astrocyte population decreased in number. The lack of astrocytic support led to a reduction in neural process outgrowth from 24.0 ± 1.3 μm on Day 7 to 7.0 ± 0.1 μm on Day 10. Subsequently, purified astrocytes were studied in isolation to understand the reasons behind PVA-SG hydrogel inability to support neural network development. It was proposed that the spatially restrictive nature (or tight mesh size) of PVA-SG hydrogels limited the astrocytic actin polymerization together with a cytoplasmic-nuclear translocation of YAP over time, causing an alteration in their cell cycle. This was confirmed by the evaluation of p27/Kip1 gene that was found to be upregulated by a twofold increase in expression at both Days 7 and 10 compared to Day 3, indicating the quiescent stage of the astrocytes in PVA-SG hydrogel. Cell migration was further studied by the quantification of MMP-2 production that was negligible compared to 2D controls, ranging from 2.7 ± 2.3% on Day 3 to 5.3 ± 2.9% on Day 10. This study demonstrates the importance of understanding astrocyte-material interactions at the molecular level, with the need to address spatial constraints in the 3D hydrogel environment. These findings will inform the design of future hydrogel constructs with greater capacity for remodeling by the cell population to create space for cell migration and neural process extension.
Keywords: 3D models; cell interaction; hydrogels; mechanosensory; neural tissue engineering; primary neuroprogenitors.
Copyright © 2020 Vallejo-Giraldo, Genta, Cauvi, Goding and Green.
Figures
References
-
- Aregueta-Robles U. A. (2017). Engineering a Living Electrode: Growth of Neural Networks Within a 3D Hydrogel.
-
- Aregueta-Robles U. A., Martens P. J., Poole-Warren L. A., Green R. A. (2018). Tailoring 3D hydrogel systems for neuronal encapsulation in living electrodes. J. Poly. Sci. Part B 56 273–287. 10.1002/polb.24558 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

