Ezrin Mediates Invasion and Metastasis in Tumorigenesis: A Review
- PMID: 33240887
- PMCID: PMC7683424
- DOI: 10.3389/fcell.2020.588801
Ezrin Mediates Invasion and Metastasis in Tumorigenesis: A Review
Abstract
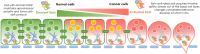
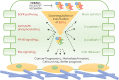
Ezrin, as encoded by the EZR gene, is a member of the Ezrin/Radixin/Moesin (ERM) family. The ERM family includes three highly related actin filament binding proteins, Ezrin, Radixin, and Moesin. These three members share similar structural properties containing an N-terminal domain named FERM, a central helical linker region, and a C-terminal domain that mediates the interaction with F-actin. Ezrin protein is highly regulated through the conformational change between a closed, inactivate form and an open, active form. As a membrane-cytoskeleton linker protein, Ezrin facilitates numerous signal transductions in tumorigenesis and mediates diverse essential functions through interactions with a variety of growth factor receptors and adhesion molecules. Emerging evidence has demonstrated that Ezrin is an oncogene protein, as high levels of Ezrin are associated with metastatic behavior in various types of cancer. The diverse functions attributed to Ezrin and the understanding of how Ezrin drives the deadly process of metastasis are complex and often controversial. Here by reviewing recent findings across a wide spectrum of cancer types we will highlight the structures, protein interactions and oncogenic roles of Ezrin as well as the emerging therapeutic agents targeting Ezrin. This review provides a comprehensive framework to guide future studies of Ezrin and other ERM proteins in basic and clinical studies.
Keywords: Ezrin; cancer; invasion; metastasis; migration.
Copyright © 2020 Song, Ma, Zhang, Wang, Wang, Ye and Xia.
Figures

References
-
- Abdou A. G., Maraee A. H., El-Sayed E. M., Elnaidany N. F. (2011). Immunohistochemical expression of ezrin in cutaneous basal and squamous cell carcinomas. Ann. Diagn. Pathol. 15 394–401. - PubMed
-
- Andersson G., Wennersten C., Gaber A., Boman K., Nodin B., Uhlen M., et al. (2014). Reduced expression of ezrin in urothelial bladder cancer signifies more advanced tumours and an impaired survival: validatory study of two independent patient cohorts. BMC Urol. 14:36. 10.1186/1471-2490-14-36 - DOI - PMC - PubMed
-
- Antoine-Bertrand J., Ghogha A., Luangrath V., Bedford F. K., Lamarche-Vane N. (2011). The activation of ezrin-radixin-moesin proteins is regulated by netrin-1 through Src kinase and RhoA/Rho kinase activities and mediates netrin-1-induced axon outgrowth. Mol. Biol. Cell 22 3734–3746. 10.1091/mbc.e10-11-0917 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

