The Secretive Life of Neutrophils Revealed by Intravital Microscopy
- PMID: 33240898
- PMCID: PMC7683517
- DOI: 10.3389/fcell.2020.603230
The Secretive Life of Neutrophils Revealed by Intravital Microscopy
Abstract
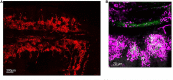
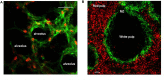
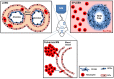
Neutrophils are the most abundant circulating leukocyte within the blood stream and for many years the dogma has been that these cells migrate rapidly into tissues in response to injury or infection, forming the first line of host defense. While it has previously been documented that neutrophils marginate within the vascular beds of the lung and liver and are present in large numbers within the parenchyma of tissues, such as spleen, lymph nodes, and bone marrow (BM), the function of these tissue resident neutrophils under homeostasis, in response to pathogen invasion or injury has only recently been explored, revealing the unexpected role of these cells as immunoregulators or immune helpers and also unraveling their heterogeneity and plasticity. Neutrophils are highly motile cells and the use of intravital microscopy (IVM) to image cells within their environment with little manipulation has dramatically increased our understanding of the function, migratory behavior, and interaction of these short-lived cells with other innate and adaptive immune cells. Contrary to previous dogma, these studies have shown that marginated and tissue resident neutrophils are the first responders to pathogens and injury, critical in limiting the spread of infection and contributing to the orchestration of the subsequent immune response. The interplay of neutrophils, with other neutrophils, leukocytes, and stroma cells can also modulate and tune their early and late response in order to eradicate pathogens, minimize tissue damage, and, in certain circumstances, contribute to tissue repair. In this review, we will follow the extraordinary journey of neutrophils from their origin in the BM to their death, exploring their role as tissue resident cells in the lung, spleen, lymph nodes, and skin and outlining the importance of neutrophil subsets, their functions under homeostasis, and in response to infection. Finally, we will comment on how understanding these processes in greater detail at a molecular level can lead to development of new therapeutics.
Keywords: intravital microscopy; neutrophil migration; neutrophil mobilization; neutrophil subsets/heterogeneity; tissue resident neutrophils.
Copyright © 2020 De Filippo and Rankin.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

