Emerging Role of Adipocyte Dysfunction in Inducing Heart Failure Among Obese Patients With Prediabetes and Known Diabetes Mellitus
- PMID: 33240938
- PMCID: PMC7667132
- DOI: 10.3389/fcvm.2020.583175
Emerging Role of Adipocyte Dysfunction in Inducing Heart Failure Among Obese Patients With Prediabetes and Known Diabetes Mellitus
Abstract
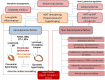
Adipose tissue dysfunction is a predictor for cardiovascular (CV) events and heart failure (HF) in patient population with obesity, metabolic syndrome, and known type 2 diabetes mellitus. Previous preclinical and clinical studies have yielded controversial findings regarding the role of accumulation of adipose tissue various types in CV risk and HF-related clinical outcomes in obese patients. There is evidence for direct impact of infiltration of epicardial adipocytes into the underlying myocardium to induce adverse cardiac remodeling and mediate HF development and atrial fibrillation. Additionally, perivascular adipocytes accumulation is responsible for release of proinflammatory adipocytokines (adiponectin, leptin, resistin), stimulation of oxidative stress, macrophage phenotype switching, and worsening vascular reparation, which all lead to microvascular inflammation, endothelial dysfunction, atherosclerosis acceleration, and finally to increase in CV mortality. However, systemic effects of white and brown adipose tissue can be different, and adipogenesis including browning of adipose tissue and deficiency of anti-inflammatory adipocytokines (visfatin, omentin, zinc-α2-glycoprotein, glypican-4) was frequently associated with adipose triglyceride lipase augmentation, altered glucose homeostasis, resistance to insulin of skeletal muscles, increased cardiomyocyte apoptosis, lowered survival, and weak function of progenitor endothelial cells, which could significantly influence on HF development, as well as end-organ fibrosis and multiple comorbidities. The exact underlying mechanisms for these effects are not fully understood, while they are essential to help develop improved treatment strategies. The aim of the review is to summarize the evidence showing that adipocyte dysfunction may induce the onset of HF and support advance of HF through different biological mechanisms involving inflammation, pericardial, and perivascular adipose tissue accumulation, adverse and electrical cardiac remodeling, and skeletal muscle dysfunction. The unbalancing effects of natriuretic peptides, neprilysin, and components of renin-angiotensin system, as exacerbating cause of altered adipocytokine signaling on myocardium and vasculature, in obesity patients at high risk of HF are disputed. The profile of proinflammatory and anti-inflammatory adipocytokines as promising biomarker for HF risk stratification is discussed in the review.
Keywords: adipose tissue; biomarkers; cardiac and vascular remodeling; co-morbidities; heart failure.
Copyright © 2020 Berezin, Berezin and Lichtenauer.
Figures



References
-
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. (2016) 388:1545–602. 10.1016/S0140-6736(16)31678-6 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

