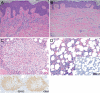
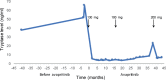
Avapritinib for Cutaneous Mastocytosis
- PMID: 33241424
- PMCID: PMC9309867
- DOI: 10.2340/00015555-3709
Avapritinib for Cutaneous Mastocytosis
Figures

Similar articles
-
Systemic mastocytosis with an associated hematologic neoplasm complicated by recurrent anaphylaxis: Prompt resolution of anaphylaxis with the addition of avapritinib.J Allergy Clin Immunol Pract. 2021 Jun;9(6):2534-2536. doi: 10.1016/j.jaip.2021.02.040. Epub 2021 Mar 4. J Allergy Clin Immunol Pract. 2021. PMID: 33677080 No abstract available.
-
Efficacy of avapritinib in refractory aggressive systemic mastocytosis: a single-patient case.Pol Arch Intern Med. 2023 May 23;133(5):16471. doi: 10.20452/pamw.16471. Epub 2023 Mar 30. Pol Arch Intern Med. 2023. PMID: 36994901 No abstract available.
-
KIT-mutated pediatric core-binding factor systemic mastocytosis-acute myeloid leukemia treated with avapritinib and decitabine.Pediatr Blood Cancer. 2024 Apr;71(4):e30898. doi: 10.1002/pbc.30898. Epub 2024 Jan 30. Pediatr Blood Cancer. 2024. PMID: 38291730 No abstract available.
-
Indirect treatment comparisons of avapritinib versus midostaurin for patients with advanced systemic mastocytosis.Future Oncol. 2022 Apr;18(13):1583-1594. doi: 10.2217/fon-2021-1509. Epub 2022 Feb 4. Future Oncol. 2022. PMID: 35114819
-
Kit Mutations: New Insights and Diagnostic Value.Immunol Allergy Clin North Am. 2018 Aug;38(3):411-428. doi: 10.1016/j.iac.2018.04.005. Epub 2018 Jun 9. Immunol Allergy Clin North Am. 2018. PMID: 30007460 Review.
Cited by
-
Mastocytosis and Mast Cell Activation Disorders: Clearing the Air.Int J Mol Sci. 2021 Oct 19;22(20):11270. doi: 10.3390/ijms222011270. Int J Mol Sci. 2021. PMID: 34681933 Free PMC article. Review.
References
-
- Erben P, Schwaab J, Metzgeroth G, Horny HP, Jawhar M, Sotlar K, et al. . The KIT D816V expressed allele burden for diagnosis and disease monitoring of systemic mastocytosis. Ann Hematol 2014; 93: 81–88. - PubMed
-
- Severino M, Chandesris MO, Barete S, Tournier E, Sans B, Laurent C, et al. . Telangiectasia macularis eruptiva perstans (TMEP): A form of cutaneous mastocytosis with potential systemic involvement. J Am Acad Dermatol 2016; 74: 885–891 e881. - PubMed
-
- Evans EK, Gardino AK, Kim JL, Hodous BL, Shutes A, Davis A, et al. . A precision therapy against cancers driven by KIT/PDGFRA mutations. Sci Transl Med 2017; 9: eaao1690. - PubMed
-
- Drummond MW, DeAngelo DJ, Deininger MW, Radia D, Quiery AT, Hexner EO, et al. . Preliminary safety and clinical activity in a Phase 1 study of Blu-285, a potent, highly-selective inhibitor of KIT D816V in advanced systemic mastocytosis (SM). Blood 2016; 128: 477–477.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

