Exenatide-loaded inside-porous poly(lactic-co-glycolic acid) microspheres as a long-acting drug delivery system with improved release characteristics
- PMID: 33241694
- PMCID: PMC7875555
- DOI: 10.1080/10717544.2020.1850919
Exenatide-loaded inside-porous poly(lactic-co-glycolic acid) microspheres as a long-acting drug delivery system with improved release characteristics
Abstract
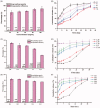
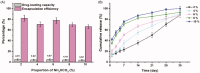
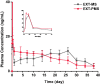
The glucagon-like peptide-1 receptor agonist exenatide (EXT) is an effective treatment for type 2 diabetes. However, this peptide has a short biological half-life and the delayed release characteristic of current formulations limit its clinical application. Herein, we prepared EXT-loaded inside-porous poly(d,l-lactic-co-glycolic acid (PLGA) microspheres with outside layers (EXT-PMS) using a W1/O/W2 emulsion method with a microfluidic technique and its fabrication and formulation conditions were systematically investigated. In vitro dissolution experiments showed that the PLGA concentration, proportion of drug and oil phase, and the number and size of pores strongly affected the release behaviors of EXT-PMS. In vitro, the optimized EXT-PMS with large internal pores exhibited rapid and stable release without a lag phase. In a rat model, subcutaneous administration of the product yielded plasma concentrations of EXT that was sustained for 30 days with low burst and no delayed-release effect. The preparation of inside-porous microspheres is lighting up the development of long-acting drug delivery systems for other drugs with favorable release characteristics.
Keywords: Exenatide; inside-porous microspheres; long-acting; release behavior; type 2 diabetes.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


References
-
- Bae SE, Son JS, Park K, Han DK. (2009). Fabrication of covered porous PLGA microspheres using hydrogen peroxide for controlled drug delivery and regenerative medicine. J Control Release 133:37–43. - PubMed
-
- Chatterjee S, Khunti K, Davies MJ. (2017). Type 2 diabetes. The Lancet 389:2239–51. - PubMed
-
- Danhier F, Ansorena E, Silva JM, et al. (2012). PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 161:505–22. - PubMed
-
- Davidson MB, Bate G, Kirkpatrick P. (2005). Exenatide. Nat Rev Drug Discov 4:713–4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
