Time course of efficacy of ubrogepant for the acute treatment of migraine: Clinical implications
- PMID: 33241721
- PMCID: PMC8047719
- DOI: 10.1177/0333102420970523
Time course of efficacy of ubrogepant for the acute treatment of migraine: Clinical implications
Abstract
Background: The full utility of an acute treatment requires examination of the entire time course of effect during a migraine attack. Here the time course of effect of ubrogepant is evaluated.
Methods: ACHIEVE-I and -II were double-blind, single-attack, Phase 3 trials. Adults with migraine were randomised 1:1:1 to placebo or ubrogepant (50mg or 100mg, ACHIEVE-I; 25 mg or 50 mg, ACHIEVE-II). Pain freedom, absence of most bothersome symptom, and pain relief were assessed at various timepoints. Samples were collected for pharmacokinetic analysis. Data were pooled for this post-hoc analysis.
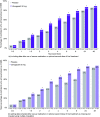
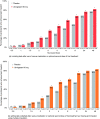
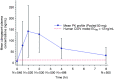
Results: Participants' (n = 912 placebo, n = 887 ubrogepant 50 mg, pooled analysis population) mean age was 41 years, with a majority female and white. Pain relief separated from placebo by 1 h (43% versus 37% [OR, 95% CI: 1.30, 1.0-1.59]), absence of most bothersome symptom by 1.5 h (28% versus 22% [1.42, 1.14-1.77]), and pain freedom by 2 h (20% vs. 13% [1.72, 1.33-2.22]). Efficacy was sustained from 2-24 h (pain relief: 1.71, 1.1-2.6; pain freedom: 1.71, 1.3-2.3) and remained separated at 48 h (pain relief: 1.7, 1.1-2.6; pain freedom: 1.31, 1.0-1.7). Pharmacokinetic analysis demonstrated maximum plasma concentrations were achieved at 1 h, with pharmacologically active concentrations reached within 11 min and remaining above the EC90 for nearly 12 h.
Conclusions: Evaluation of the time course of effect of ubrogepant showed pain relief as the most sensitive and earliest measure of clinical effect, followed by absence of most bothersome symptom, and pain freedom. Efficacy was demonstrated out to 48 h, providing evidence of the long-lasting effect of ubrogepant. This evaluation supports the role of examining the entire time course of effect to understand fully the utility of an acute treatment for migraine.Trial registration: ACHIEVE I (ClinicalTrials.gov, NCT02828020) and ACHIEVE II (ClinicalTrials.gov, NCT02867709).
Keywords: Migraine; acute; calcitonin gene-related peptide.
Conflict of interest statement
AMB received fees from Allergan (now AbbVie), Aeon, Amgen, Alder, Biohaven, Eli Lilly and Company, ElectroCore, Novartis, Revance, Theranica, and Teva Pharmaceuticals.
RBL reports the following conflicts from within the past 48 months: Serves on the editorial boards of
DWD reports consulting: Amgen, Association of Translational Medicine, University Health Network, Daniel Edelman Inc., Autonomic Technologies, Axsome, Aural Analytics, Allergan (now AbbVie), Alder BioPharmaceuticals, Biohaven, Charleston Laboratories, Dr Reddy's Laboratories/Promius, Electrocore LLC, Eli Lilly, eNeura, Neurolief, Novartis, Ipsen, Impel, Satsuma, Supernus, Sun Pharma (India), Theranica, Teva, Vedanta, WL Gore, Nocira, PSL Group Services, University of British Columbia, XoC, Zosano, ZP Opco, Foresite Capital, Oppenheimer; Upjohn (Division of Pfizer), Pieris, Revance, Equinox, Salvia, Amzak Health. CME fees or royalty payments: HealthLogix, Medicom Worldwide, MedLogix Communications, Mednet, Miller Medical, PeerView, WebMD Health/Medscape, Chameleon, Academy for Continued Healthcare Learning, Universal Meeting Management, Haymarket, Global Scientific Communications, Global Life Sciences, Global Access Meetings, UpToDate (Elsevier), Oxford University Press, Cambridge University Press, Wolters Kluwer Health; Stock options: Precon Health, Aural Analytics, Healint, Theranica, Second Opinion/Mobile Health, Epien, GBS/Nocira, Matterhorn/Ontologics, King-Devick Technologies; Consulting without fee: Aural Analytics, Healint, Second Opinion/Mobile Health, Epien; Precon Health, Board of Directors: Epien, Matterhorn/Ontologics, King-Devick Technologies. Patent: 17189376.1-1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis without fee; research funding: American Migraine Foundation, US Department of Defense, PCORI, Henry Jackson Foundation; Professional society fees or reimbursement for travel: American Academy of Neurology, American Brain Foundation, American Headache Society, American Migraine Foundation, International Headache Society, Canadian Headache Society.
KK received personal fees from Amgen, Eli Lilly and Company, Allergan (now AbbVie), Novartis, and Theranica.
AMA is a full-time employee and stockholder of AbbVie.
AJ was a full-time employee and stockholder of AbbVie at the time of the trial conduct and drafting of the manuscript.
CL is a full-time employee and stockholder of AbbVie.
AS was a full-time employee and stockholder of AbbVie at the time of the trial conduct and drafting of the manuscript.
JMT is a full-time employee and stockholder of AbbVie.
Figures


References
-
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015; 55: 3–20. - PubMed
-
- Ferrari MD, Roon KI, Lipton RB, et al.. Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: A meta-analysis of 53 trials. Lancet 2001; 358: 1668–1675. - PubMed
-
- Tfelt-Hansen P, Saxena PR, Dahlof C, et al.. Ergotamine in the acute treatment of migraine: A review and European consensus. Brain 2000; 123: 9–18. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

