The development of proteinase-activated receptor-2 modulators and the challenges involved
- PMID: 33242065
- PMCID: PMC7752072
- DOI: 10.1042/BST20200191
The development of proteinase-activated receptor-2 modulators and the challenges involved
Abstract
Protease-activated receptor-2 (PAR2) has been extensively studied since its discovery in the mid-1990. Despite the advances in understanding PAR2 pharmacology, it has taken almost 25 years for the first inhibitor to reach clinical trials, and so far, no PAR2 antagonist has been approved for human use. Research has employed classical approaches to develop a wide array of PAR2 agonists and antagonists, consisting of peptides, peptoids and antibodies to name a few, with a surge in patent applications over this period. Recent breakthroughs in PAR2 structure determination has provided a unique insight into proposed PAR2 ligand binding sites. Publication of the first crystal structures of PAR2 resolved in complex with two novel non-peptide small molecule antagonists (AZ8838 and AZ3451) revealed two distinct binding pockets, originally presumed to be allosteric sites, with a PAR2 antibody (Fab3949) used to block tethered ligand engagement with the peptide-binding domain of the receptor. Further studies have proposed orthosteric site occupancy for AZ8838 as a competitive antagonist. One company has taken the first PAR2 antibody (MEDI0618) into phase I clinical trial (NCT04198558). While this first-in-human trial is at the early stages of the assessment of safety, other research into the structural characterisation of PAR2 is still ongoing in an attempt to identify new ways to target receptor activity. This review will focus on the development of novel PAR2 modulators developed to date, with an emphasis placed upon the advances made in the pharmacological targeting of PAR2 activity as a strategy to limit chronic inflammatory disease.
Keywords: PARs; inflammation; modulators.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
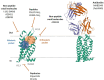
References
-
- Macfarlane S.R., Seatter M.J., Kanke T., Hunter G.D. and Plevin R. (2001) Proteinase-activated receptors. Pharmacol. Rev. 53, 245–282 PMID: - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

