Riociguat: Clinical research and evolving role in therapy
- PMID: 33242341
- PMCID: PMC8359233
- DOI: 10.1111/bcp.14676
Riociguat: Clinical research and evolving role in therapy
Abstract
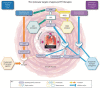
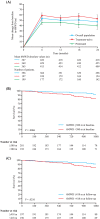
Riociguat is a first-in-class soluble guanylate cyclase stimulator, approved for the treatment of adults with pulmonary arterial hypertension (PAH), inoperable chronic thromboembolic pulmonary hypertension (CTEPH), or persistent or recurrent CTEPH after pulmonary endarterectomy. Approval was based on the results of the phase III PATENT-1 (PAH) and CHEST-1 (CTEPH) studies, with significant improvements in the primary endpoint of 6-minute walk distance vs placebo of +36 m and +46 m, respectively, as well as improvements in secondary endpoints such as pulmonary vascular resistance and World Health Organization functional class. Riociguat acts as a stimulator of cyclic guanosine monophosphate synthesis rather than as an inhibitor of cGMP metabolism. As with other approved therapies for PAH, riociguat has antifibrotic, antiproliferative and anti-inflammatory effects, in addition to vasodilatory properties. This has led to further clinical studies in patients who do not achieve a satisfactory clinical response with phosphodiesterase type-5 inhibitors. Riociguat has also been evaluated in patients with World Health Organization group 2 and 3 pulmonary hypertension, and other conditions including diffuse cutaneous systemic sclerosis, Raynaud's phenomenon and cystic fibrosis. This review evaluates the results of the original clinical trials of riociguat for the treatment of PAH and CTEPH, and summarises the body of work that has examined the safety and efficacy of riociguat for the treatment of other types of pulmonary hypertension.
Keywords: drug information; pharmacotherapy; therapeutics.
© 2020 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society..
Conflict of interest statement
J.R.K. reports research support to his institution from Actelion, Bayer AG, Lung Biotechnology and United Therapeutics. M.M.C. has received research support from Actelion, Eiger BioPharmaceuticals, GeNO LLC, Gilead, GlaxoSmithKline, Medtronic and Reata Pharmaceuticals; consulting fees from Actelion, Express Scripts Holding Company, Gilead, SteadyMed Therapeutics, United Therapeutics and WebMD LLC (Medscape); and honoraria for speaking for Bayer AG and Gilead. D.L. reports honoraria, consultation fees, research support and/or travel expenses from Actelion, Arena, Bayer AG, Northern Therapeutics, PhaseBio and United Therapeutics. S.R. reports grants and personal fees from Abbott, Actelion, Arena, Bayer AG, Ferrer, Gilead, GlaxoSmithKline, MSD, Novartis, Pfizer and United Therapeutics, and research support from Actelion, Bayer, Novartis, Pfizer and United Therapeutics. O.S. reports grants, personal fees and nonfinancial support from Actelion, Bayer AG, GlaxoSmithKline and Merck, and personal fees from Arena.
Figures


References
-
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67‐119. - PubMed
-
- Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D92‐D99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

