X-aptamers targeting Thy-1 membrane glycoprotein in pancreatic ductal adenocarcinoma
- PMID: 33242496
- PMCID: PMC7863625
- DOI: 10.1016/j.biochi.2020.11.018
X-aptamers targeting Thy-1 membrane glycoprotein in pancreatic ductal adenocarcinoma
Abstract
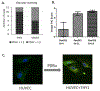
Modified DNA aptamers incorporated with amino-acid like side chains or drug-like ligands can offer unique advantages and enhance specificity as affinity ligands. Thy-1 membrane glycoprotein (THY1 or CD90) was previously identified as a biomarker candidate of neovasculature in pancreatic ductal adenocarcinoma (PDAC). The current study developed and evaluated modified DNA X-aptamers targeting THY1 in PDAC. The expression and glycosylation of THY1 in PDAC tumor tissues were assessed using immunohistochemistry and quantitative proteomics. Bead-based X-aptamer library that contains 108 different sequences was used to screen for high affinity THY1 X-aptamers. The sequences of the X-aptamers were analyzed with the next-generation sequencing. The affinities of the selected X-aptamers to THY1 were quantitatively evaluated with flow cytometry. Three high affinity THY1 X-aptamers, including XA-B217, XA-B216 and XA-A9, were selected after library screening and affinity binding evaluation. These three X-aptamers demonstrated a high binding affinity and specificity to THY1 protein and the THY1 expressing cell lines, using THY1 antibody as a comparison. The development of these X-aptamers provides highly specific and non-immunogenic affinity ligands for THY1 binding in the context of biomarker development and clinical applications. They could be further exploited to assist molecular imaging of PDAC targeting THY1.
Keywords: Aptamer; Pancreatic cancer; Proteomics; Thy-1 membrane glycoprotein (THY1 or CD90).
Copyright © 2020 Elsevier B.V. and Société Française de Biochimie et Biologie Moléculaire (SFBBM). All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

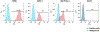





References
-
- Saalbach A, et al. , The Thy-1/Thy-1 ligand interaction is involved in binding of melanoma cells to activated Thy-1- positive microvascular endothelial cells. Microvasc Res, 2002. 64(1): p. 86–93. - PubMed
-
- Hermann PC, et al. , Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell, 2007. 1(3): p. 313–23. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

