Automation and data-driven design of polymer therapeutics
- PMID: 33242537
- PMCID: PMC8127395
- DOI: 10.1016/j.addr.2020.11.009
Automation and data-driven design of polymer therapeutics
Abstract
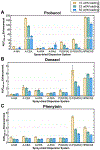
Polymers are uniquely suited for drug delivery and biomaterial applications due to tunable structural parameters such as length, composition, architecture, and valency. To facilitate designs, researchers may explore combinatorial libraries in a high throughput fashion to correlate structure to function. However, traditional polymerization reactions including controlled living radical polymerization (CLRP) and ring-opening polymerization (ROP) require inert reaction conditions and extensive expertise to implement. With the advent of air-tolerance and automation, several polymerization techniques are now compatible with well plates and can be carried out at the benchtop, making high throughput synthesis and high throughput screening (HTS) possible. To avoid HTS pitfalls often described as "fishing expeditions," it is crucial to employ intelligent and big data approaches to maximize experimental efficiency. This is where the disruptive technologies of machine learning (ML) and artificial intelligence (AI) will likely play a role. In fact, ML and AI are already impacting small molecule drug discovery and showing signs of emerging in drug delivery. In this review, we present state-of-the-art research in drug delivery, gene delivery, antimicrobial polymers, and bioactive polymers alongside data-driven developments in drug design and organic synthesis. From this insight, important lessons are revealed for the polymer therapeutics community including the value of a closed loop design-build-test-learn workflow. This is an exciting time as researchers will gain the ability to fully explore the polymer structural landscape and establish quantitative structure-property relationships (QSPRs) with biological significance.
Keywords: Artificial intelligence; Automation; Drug delivery; Gene delivery; High throughput screening; Machine learning; Polymer chemistry.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures


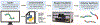






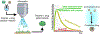
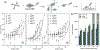


References
-
- Rudra A, Li J, Shakur R, Bhagchandani S, Langer R, Trends in Therapeutic Conjugates: Bench to Clinic, Bioconjug. Chem, 31 (2020) 462–473. - PubMed
-
- Du AW, Stenzel MH, Drug Carriers for the Delivery of Therapeutic Peptides, Biomacromolecules, 15 (2014) 1097–1114. - PubMed
-
- Russo D, de Angelis A, Garvey CJ, Wurm FR, Appavou MS, Prevost S, Effect of Polymer Chain Density on Protein-Polymer Conjugate Conformation, Biomacromolecules, 20 (2019) 1944–1955. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

