Useful road maps: studying Drosophila larva's central nervous system with the help of connectomics
- PMID: 33242722
- PMCID: PMC7773133
- DOI: 10.1016/j.conb.2020.09.008
Useful road maps: studying Drosophila larva's central nervous system with the help of connectomics
Abstract
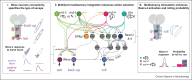
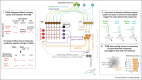
The larva of Drosophila melanogaster is emerging as a powerful model system for comprehensive brain-wide understanding of the circuit implementation of neural computations. With an unprecedented amount of tools in hand, including synaptic-resolution connectomics, whole-brain imaging, and genetic tools for selective targeting of single neuron types, it is possible to dissect which circuits and computations are at work behind behaviors that have an interesting level of complexity. Here we present some of the recent advances regarding multisensory integration, learning, and action selection in Drosophila larva.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Vosshall L.B., Stocker R.F. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. - PubMed
-
- Swanson L.W. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. - PubMed
-
- Watabe-Uchida M., Uchida N. Multiple dopamine systems: weal and woe of dopamine. Cold Spring Harb Symp Quant Biol. 2018;83:83–95. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

