Analysis of the efficacy of HIV protease inhibitors against SARS-CoV-2's main protease
- PMID: 33243253
- PMCID: PMC7689640
- DOI: 10.1186/s12985-020-01457-0
Analysis of the efficacy of HIV protease inhibitors against SARS-CoV-2's main protease
Abstract
Background: The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in millions of infections worldwide. While the search for an effective antiviral is still ongoing, experimental therapies based on repurposing of available antivirals is being attempted, of which HIV protease inhibitors (PIs) have gained considerable interest. Inhibition profiling of the PIs directly against the viral protease has never been attempted in vitro, and while few studies reported an efficacy of lopinavir and ritonavir in SARS-CoV-2 context, the mechanism of action of the drugs remains to be validated.
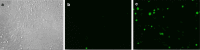
Methods: We carried out an in-depth analysis of the efficacy of HIV PIs against the main protease of SARS-CoV-2 (Mpro) in cell culture and in vitro enzymatic assays, using a methodology that enabled us to focus solely on any potential inhibitory effects of the inhibitors against the viral protease. For cell culture experiments a dark-to-bright GFP reporter substrate system was designed.
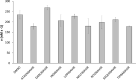
Results: Lopinavir, ritonavir, darunavir, saquinavir, and atazanavir were able to inhibit the viral protease in cell culture, albeit in concentrations much higher than their achievable plasma levels, given their current drug formulations. While inhibition by lopinavir was attributed to its cytotoxicity, ritonavir was the most effective of the panel, with IC50 of 13.7 µM. None of the inhibitors showed significant inhibition of SARS-CoV-2 Mpro in our in vitro enzymatic assays up to 100 µM concentration.
Conclusion: Targeting of SARS-CoV-2 Mpro by some of the HIV PIs might be of limited clinical potential, given the high concentration of the drugs required to achieve significant inhibition. Therefore, given their weak inhibition of the viral protease, any potential beneficial effect of the PIs in COVID-19 context might perhaps be attributed to acting on other molecular target(s), rather than SARS-CoV-2 Mpro.
Keywords: HIV protease inhibitors; In vitro assay; Inhibition profiling; Protease; SARS-CoV-2.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

