Epigenetic modification mechanisms involved in keloid: current status and prospect
- PMID: 33243301
- PMCID: PMC7690154
- DOI: 10.1186/s13148-020-00981-8
Epigenetic modification mechanisms involved in keloid: current status and prospect
Abstract
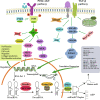
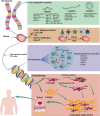
Keloid, a common dermal fibroproliferative disorder, is benign skin tumors characterized by the aggressive fibroblasts proliferation and excessive accumulation of extracellular matrix. However, common therapeutic approaches of keloid have limited effectiveness, emphasizing the momentousness of developing innovative mechanisms and therapeutic strategies. Epigenetics, representing the potential link of complex interactions between genetics and external risk factors, is currently under intense scrutiny. Accumulating evidence has demonstrated that multiple diverse and reversible epigenetic modifications, represented by DNA methylation, histone modification, and non-coding RNAs (ncRNAs), play a critical role in gene regulation and downstream fibroblastic function in keloid. Importantly, abnormal epigenetic modification manipulates multiple behaviors of keloid-derived fibroblasts, which served as the main cellular components in keloid skin tissue, including proliferation, migration, apoptosis, and differentiation. Here, we have reviewed and summarized the present available clinical and experimental studies to deeply investigate the expression profiles and clarify the mechanisms of epigenetic modification in the progression of keloid, mainly including DNA methylation, histone modification, and ncRNAs (miRNA, lncRNA, and circRNA). Besides, we also provide the challenges and future perspectives associated with epigenetics modification in keloid. Deciphering the complicated epigenetic modification in keloid is hopeful to bring novel insights into the pathogenesis etiology and diagnostic/therapeutic targets in keloid, laying a foundation for optimal keloid ending.
Keywords: DNA methylation; Epigenetic modification; Histone modification; Keloid; ncRNAs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Saveria Fioretto B, Rosa I, Romano E, Wang Y, Guiducci S, Zhang G, Manetti M, Matucci-Cerinic M. The contribution of epigenetics to the pathogenesis and gender dimorphism of systemic sclerosis: a comprehensive overview. Ther Adv Musculoskelet Dis. 2020;12:1759720X20918456. doi: 10.1177/1759720X20918456. - DOI - PMC - PubMed
-
- Jumper N, Paus R, Bayat A. Functional histopathology of keloid disease. Histol Histopathol. 2015;30(9):1033–1057. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

