4D-DSA: Development and Current Neurovascular Applications
- PMID: 33243899
- PMCID: PMC7872169
- DOI: 10.3174/ajnr.A6860
4D-DSA: Development and Current Neurovascular Applications
Abstract
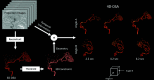
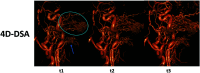
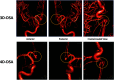
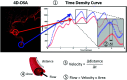
Originally described by Davis et al in 2013, 4D-Digital Subtraction Angiography (4D-DSA) has developed into a commercially available application of DSA in the angiography suite. 4D-DSA provides the user with 3D time-resolved images, allowing observation of a contrast bolus at any desired viewing angle through the vasculature and at any time point during the acquisition (any view at any time). 4D-DSA mitigates some limitations that are intrinsic to both 2D- and 3D-DSA images. The clinical applications for 4D-DSA include evaluations of AVMs and AVFs, intracranial aneurysms, and atherosclerotic occlusive disease. Recent advances in blood flow quantification using 4D-DSA indicate that these data provide both the velocity and geometric information necessary for the quantification of blood flow. In this review, we will discuss the development, acquisition, reconstruction, and current neurovascular applications of 4D-DSA volumes.
© 2021 by American Journal of Neuroradiology.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
