Head and neck squamous cell carcinoma
- PMID: 33243986
- PMCID: PMC7944998
- DOI: 10.1038/s41572-020-00224-3
Head and neck squamous cell carcinoma
Erratum in
-
Author Correction: Head and neck squamous cell carcinoma.Nat Rev Dis Primers. 2023 Jan 19;9(1):4. doi: 10.1038/s41572-023-00418-5. Nat Rev Dis Primers. 2023. PMID: 36658129 No abstract available.
Abstract
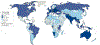
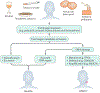
Most head and neck cancers are derived from the mucosal epithelium in the oral cavity, pharynx and larynx and are known collectively as head and neck squamous cell carcinoma (HNSCC). Oral cavity and larynx cancers are generally associated with tobacco consumption, alcohol abuse or both, whereas pharynx cancers are increasingly attributed to infection with human papillomavirus (HPV), primarily HPV-16. Thus, HNSCC can be separated into HPV-negative and HPV-positive HNSCC. Despite evidence of histological progression from cellular atypia through various degrees of dysplasia, ultimately leading to invasive HNSCC, most patients are diagnosed with late-stage HNSCC without a clinically evident antecedent pre-malignant lesion. Traditional staging of HNSCC using the tumour-node-metastasis system has been supplemented by the 2017 AJCC/UICC staging system, which incorporates additional information relevant to HPV-positive disease. Treatment is generally multimodal, consisting of surgery followed by chemoradiotherapy (CRT) for oral cavity cancers and primary CRT for pharynx and larynx cancers. The EGFR monoclonal antibody cetuximab is generally used in combination with radiation in HPV-negative HNSCC where comorbidities prevent the use of cytotoxic chemotherapy. The FDA approved the immune checkpoint inhibitors pembrolizumab and nivolumab for treatment of recurrent or metastatic HNSCC and pembrolizumab as primary treatment for unresectable disease. Elucidation of the molecular genetic landscape of HNSCC over the past decade has revealed new opportunities for therapeutic intervention. Ongoing efforts aim to integrate our understanding of HNSCC biology and immunobiology to identify predictive biomarkers that will enable delivery of the most effective, least-toxic therapies.
Conflict of interest statement
Competing interests
D.E.J. and J.R.G. are co-inventors of cyclic STAT3 decoy and have financial interests in STAT3 Therapeutics. STAT3 Therapeutics holds an interest in cyclic STAT3 decoy. B.A.B. has received honoraria for consulting from Merck and AstraZeneca. C.R.L. serves on the Advisory Board of Merck & Co. and Rakuten Medical. V.W.Y.L. receives grant support from Lee’s Pharmaceutical, Hong Kong Limited, via the University-Industry Collaboration Program (UIM/329; from the Innovation and Technology Fund, Hong Kong government; in 2018–2020), and served as a scientific consultant for Novartis Pharmaceutical (Hong Kong) Limited (Oct 2015-Oct 2016). J.E.B. serves as a scientific consultant to CUE Pharmaceuticals and Astra Zeneca and has research grant support from the IST programs of Aveo and Novartis.
Figures
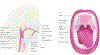
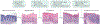


References
-
- Bonner JA et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354, 567–578 (2006). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

