Plasma imatinib levels and ABCB1 polymorphism influences early molecular response and failure-free survival in newly diagnosed chronic phase CML patients
- PMID: 33244077
- PMCID: PMC7691501
- DOI: 10.1038/s41598-020-77140-9
Plasma imatinib levels and ABCB1 polymorphism influences early molecular response and failure-free survival in newly diagnosed chronic phase CML patients
Abstract
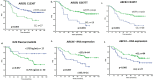
Achieving early molecular response (EMR) has been shown to be associated with better event free survival in patients with chronic phase chronic myeloid leukemia (CP-CML) on Imatinib therapy. We prospectively evaluated the factors influencing the 2-year failure free survival (FFS) and EMR to imatinib therapy in these patients including day29 plasma Imatinib levels, genetic variants and the gene expression of target genes in imatinib transport and biotransformation. Patients with low and intermediate Sokal score had better 2-year FFS compared to those with high Sokal Score (p = 0.02). Patients carrying ABCB1-C1236T variants had high day29 plasma imatinib levels (P = 0.005), increased EMR at 3 months (P = 0.044) and a better 2 year FFS (P = 0.003) when compared to those with wild type genotype. This translates to patients with lower ABCB1 mRNA expression having a significantly higher intracellular imatinib levels (P = 0.029). Higher day29 plasma imatinib levels was found to be strongly associated with patients achieving EMR at 3 months (P = 0.022), MMR at 12 months (P = 0.041) which essentially resulted in better 2-year FFS (p = 0.05). Also, patients who achieved EMR at 3 months, 6 months and MMR at 12 months had better FFS when compared to those who did not. This study suggests the incorporation of these variables in to the imatinib dosing algorithm as predictive biomarkers of response to Imatinib therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

